May 21st, 2009 by Dr. Val Jones in Health Tips, True Stories
No Comments »
There’s no technological substitute for the human hand. Manual dexterity is incredibly hard to replicate, and so surgeons will go to great lengths to save injured hands. Unfortunately, sometimes the injury is too severe to allow for any meaningful functional recovery.
In these two cases, well-meaning surgeons refused to amputate the unsalvageable hands, thus delaying recovery and adaptation of prostheses.
This is a photo of a trauma victim who underwent extensive reconstruction of the hand, including transplantation of a toe to the thumb’s position. Gangrene set in and tracked up one of the tendon sheaths.
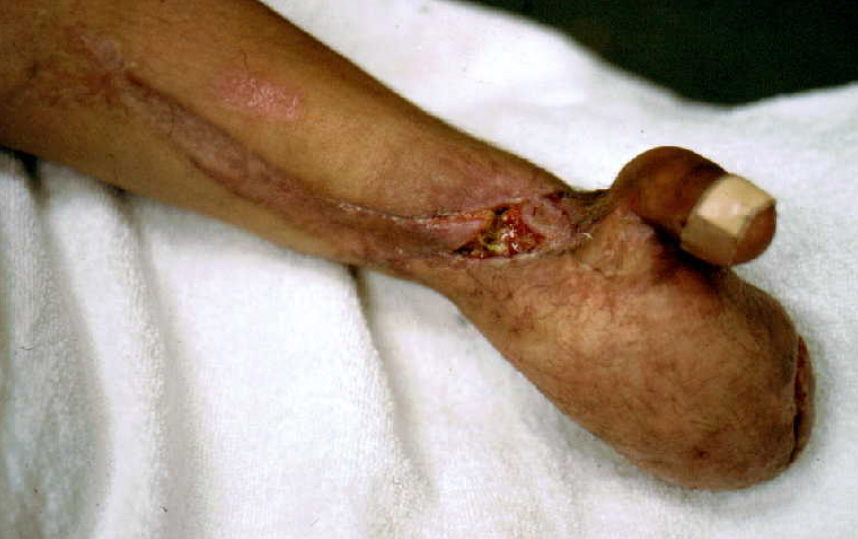
Photo Credit: Dr. Heikki Uustal
In this case, a burn victim was hoping to have some fingers reconstructed from his fist. He declined amputation and fitting with a prosthesis, despite the potential for enhanced function.
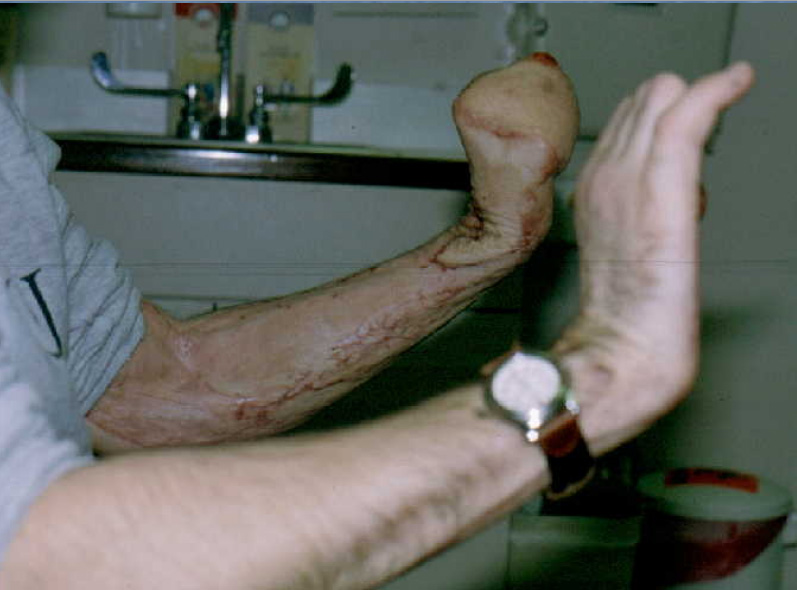
Photo Credit: Dr. Heikki Uustal
In both cases, a wrist disarticulation (amputation at the wrist) and prosthetic fitting (such as this myo-electric device with a self-suspending socket) might have provided a better functional and cosmetic outcome:
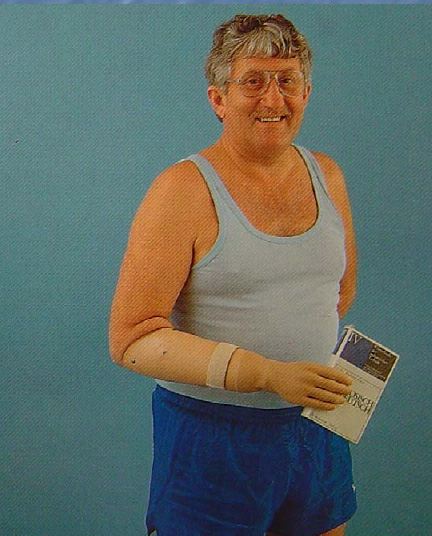
Photo Credit: Dr. Heikki Uustal
Sometimes, it’s better to amputate.
March 4th, 2009 by Dr. Val Jones in Audio, Expert Interviews
No Comments »

Bert Rein
The New York Times has called today’s US Supreme Court ruling in the Wyeth vs. Levine suit the “most important business case in years.” I have been following this case for many months, astonished that a medical malpractice suit had gotten all the way to the Supreme Court. But even more shocking is the fact that the court actually ruled that lay juries may evaluate the accuracy of FDA-approved drug labels written for healthcare professionals.
In other words, after a team of FDA regulators decide on the very best language to describe potential risks of a drug – Joe Six Pack can overrule their expertise and hold the drug company liable for any deficit (as he interprets it) in label language, awarding millions to anyone who experiences harm, no matter how well disclosed that risk is.
I reached out to Wyeth’s attorney, Bert Rein, for comment. Here’s a podcast of our interview:
[Audio:http://blog.getbetterhealth.com/wp-content/uploads/2009/03/wyethvlevine.mp3]
Here are the highlights from the interview…
Dr. Val: The New York Times is calling Wyeth vs. Levine the most important business case in years. Can you summarize what just happened?
Rein: The court determined that Wyeth’s liability for Ms. Levine’s injury was not preempted by the FDA-approved drug label warnings. They were not convinced that the FDA had declined to strengthen the warning language on the label prior to Ms. Levine’s injury, though Wyeth had in fact requested a label change. In addition, the court held that the FDA’s regulatory regime was insufficient to preempt Ms. Levine from suing Wyeth, because the FDA doesn’t have a regulational requirement for all label updates to undergo federal approval. The court therefore ruled that the suit was well founded and that the state of Vermont should decide whether or not Wyeth’s conduct was appropriate.
Dr. Val: So basically this means that juries can decide whether or not a drug label is sufficiently caveated?
Rein: It goes farther than that. Juries don’t have to determine what the label should say, they merely have to decide that the label isn’t “good enough.”
Dr. Val: So jurors without any medical background are supposed to determine whether or not a drug label offers physicians sufficient warning about medication risks?
Rein: Correct. You’re asking lay people not only to make the decision, but to step into the shoes of physicians and say, “Do I think that label is good enough from a physician’s point of view?” By definition, drug labels are not written for lay people, but healthcare professionals. This is asking a lot of lay people, and I think this case is a good illustration of why juries get it wrong. They see an injured person and say “How could the labeling be adequate because somebody’s been hurt?”
Dr. Val: What impact will this court ruling have on the pharmaceutical industry?
Rein: It means that pharmaceutical companies will have to get “clear records” from the FDA on every drug label controversy going forward. This puts a tremendous burden on their already taxed resources. Also if juries can simply say “this drug label is inadequate” then how will the drug company know how to make it better? What drug companies will have to do is forbid the administration of drugs in circumstance that might incur increased risk. That shifts liability to the physician if they administer the drug outside of the prescribed method – and essentially makes the risk benefit decisions on their behalf.
Dr. Val: So won’t drug companies have to create really long drug inserts to prevent juries from misunderstanding the language?
Rein: Yes, that’s the direction that labels were going before the FDA tried to reform the system. When drug labels are that long, no one reads them. Then professionals really don’t get educated on the true risks and benefits of the drug. Long labels are not designed for provider education but for law suits. Jury dominance always results in risk aversion.
Dr. Val: And isn’t this risk aversion going to slow down the drug approval process in general?
Rein: The industry shies away from developing drugs that have massive liability. That’s why we don’t develop drugs for pregnant women, for example. Any time you unleash a potent liability system, it’s going to factor in to where research dollars are spent. The more the FDA is criticized, the more it tries to protect itself with long drug labels – which ends up slowing down the drug approval process and shifting liability to doctors.
Dr. Val: And phenergan has been safely administered over 200 million times… and so the risk aversion is pretty high, even now with this rather safe drug.
Rein: Right, it’s not as if the drug is rampantly causing injury. Twenty incidents out of 200 million applications is not a very high risk profile. And the few cases where it caused injury, the drug was administered incorrectly. But if you have an injured person sitting in front of a jury of lay people, it seems as if the logical conclusion is that if the warnings were adequate, this wouldn’t have happened.
If we take the American Foundation for Justice at its word, their next move is to try to change the law on medical devices so we can go after those as well. The Wyeth vs. Levine case is good for one industry – the lawsuit industry – and not really anyone else.
###
The Supreme Court decision text may be found here.
November 6th, 2008 by Dr. Val Jones in Opinion
3 Comments »
Wyeth vs. Levine is an important legal case being tried before the US Supreme Court. You may have read about the lawsuit in the New York Times, NEJM, JAMA, the Wall Street Journal, or my own blog. It revolves around the tragic story of a woman (Ms. Levine) who experienced an extremely rare side effect (severe tissue damage resulting in the amputation of her right arm) because a drug was administered improperly (into an artery rather than a vein). Ms. Levine is arguing that her injury could have been avoided if the drug label had stronger warning language, and the Vermont Supreme Court ruled in her favor, awarding her $7 million. The court ruled that a jury in the state of Vermont had the right to hold Wyeth accountable for a different labeling standard than the one approved by the FDA.
The plot thickens, however, in that Wyeth’s FDA-approved label very clearly discourages injection of their drug into or near an artery, and it also describes the potential consequence (including gangrene) of such an action. The FDA approved Wyeth’s label in full knowledge of the potential risks and benefits of the drug. In fact, Wyeth asked to strengthen the language of the label before Ms. Levine was injured, and the FDA declined to make the change because label changes are based on new information about a drug’s frequency or severity of risks. Wyeth had nothing new to disclose. Read more »















