August 29th, 2011 by Elaine Schattner, M.D. in News
No Comments »

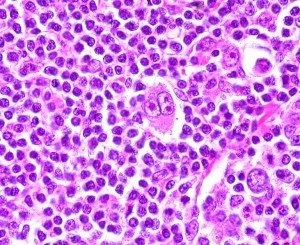 Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).
Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).
This interests me for a lot of reasons, among them that I used to work in the field of lymphoma immunology and spent some time in my life studying molecules like CD30, the protein to which the new antibody binds.
First, a mini-primer on the disease and numbers of patients involved: Read more »
*This blog post was originally published at Medical Lessons*
August 28th, 2011 by John Di Saia, M.D. in Opinion, Research
No Comments »

The FDA has granted a license to the maker of laViv which is said to improve the appearance of smile lines without freezing the muscles of your face. Have you heard of this new drug? Does it work like it claims? Are there any side effects that are worrisome?
Source: dailymail.co.uk/femail/article-2028456/New-biological-wrinkle-cure-touted-alternative-Botox-frozen-face.html
Maybe.
As we have discussed before, FDA approval is not a stamp of approval that a drug is effective. It just means that as far as current studies show, it is not harmful. Some drugs are FDA approved for years until later the FDA reconsiders and removes them from the market. Look at the relatively recent removal of Darvocet from the market after many years of FDA approval.
LaViv is an interesting concept. Read more »
*This blog post was originally published at Truth in Cosmetic Surgery*
August 4th, 2011 by AndrewSchorr in Opinion, Research
No Comments »

 There are big companies like Quintiles that run clinical trials around the world. There are local clinics that specialize in clinical trials and make a lot of money at it. There are, of course, pharmaceutical companies and device manufacturers who depend upon the results to gain marketing approval for new products. People in all those groups know a lot about trials.
There are big companies like Quintiles that run clinical trials around the world. There are local clinics that specialize in clinical trials and make a lot of money at it. There are, of course, pharmaceutical companies and device manufacturers who depend upon the results to gain marketing approval for new products. People in all those groups know a lot about trials.
But the perspective that counts is the view from you and me – patients. Most of us do not enroll in clinical trials. We don’t want to get too up close and personal with anything “experimental.” And often our doctors never tell us about available trials anyway since it can be a lot of paperwork for them. Given that most people don’t enroll in trials and new science is delayed because of it and also because most people in trials are not journalists, I thought I’d put hunt and peck to the computer keyboard and speak out about trials. I am especially motivated because I have participated twice. The first one, a leukemia trial in 2000, I believe, saved my life. And I enrolled in a second one, studying a new drug for clots in the legs (deep vein thrombosis or DVT) just a week and a half ago.
I enrolled in the DVT trial because 1) the first one worked for me and 2) I crow all the time about how patients should always consider being in a trial as a treatment option. I had to put up or shut up. So I signed on the dotted line.
This particular trial, Read more »
*This blog post was originally published at Andrew's Blog*
 Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).
Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).




 There are big companies like Quintiles that run clinical trials around the world. There are local clinics that specialize in clinical trials and make a lot of money at it. There are, of course, pharmaceutical companies and device manufacturers who depend upon the results to gain marketing approval for new products. People in all those groups know a lot about trials.
There are big companies like Quintiles that run clinical trials around the world. There are local clinics that specialize in clinical trials and make a lot of money at it. There are, of course, pharmaceutical companies and device manufacturers who depend upon the results to gain marketing approval for new products. People in all those groups know a lot about trials.







