June 9th, 2009 by RamonaBatesMD in Better Health Network, News
No Comments »

I want to say these two stories were well done (both aired on June 1, 2009). I was actually interviewed, but not quoted, for the story on fat-grafting. I pointed Allison Aubry to Dr Scott Spear as her expert. He is involved in one of the U.S. studies on breast augmentation using fat grafting.
Sculpting the Body with Recycled Fat by Allison Aubry.
Doctors Still Unsure Of Long-Term Risks
Surgeons like Dr. Scott Spear of Georgetown University Hospital want to know more about the techniques used to transfer fat for breast augmentation.
“We’re at the beginning of the learning curve,” he says. He has initiated a clinical trial to answer some questions about the best way to perform the procedure and whether there are any measurable risks. To date, there are no published studies in the United States, so doctors are relying on their own clinical experience.
Silicone Injections May Harm Some Patients by Patti Neighmond
When people get injected with silicone at pumping parties, Gorton says “there is no way to verify if they’re using medical-grade silicone. You can go to hardware stores and buy a big tub of it,” he says. “The element is the same, but it’s just not the same safety or purity or quality.”
*This blog post was originally published at Suture for a Living*
May 31st, 2009 by Dr. Val Jones in True Stories
6 Comments »
My primary care physician has a cash-only medical practice, and he is paid by the hour for whatever he does – be it a phone call, email, office visit, house call, or outpatient surgical procedure. He doesn’t charge higher prices for procedure complexity – that’s factored into the time it takes to complete the procedure. It’s a wonderful model for those of us who’ve chosen high deductible health insurance plans, and pay cash for primary care services. My husband and I save thousands of dollars/year with our plan, and spend a few hundred of that savings to cover our primary care needs. We also have our family physician available to us 24-7 via phone/email, and can generally see him for an in-person visit within hours of a request for one.
Yesterday was a perfect example of the incredible convenience of this model of care – I called Dr. Dappen at 10:30am and asked if we could come in to have a sebaceous cyst removed from my husband’s back. Dr. Dappen said he’d be happy to see us at 11:30am that day, so we hopped in a car and were finished with the procedure by 12:00. I even had fun taking photos for the blog (see below)…
Cost of the procedure: (surgery plus supplies): $150
Days spent waiting for an appointment: 0
Time spent in a waiting room: 0 minutes
Convenience of having a cash-only family physician: priceless
*For more information, check out: Doctokr Family Medicine, Vienna, Virginia*
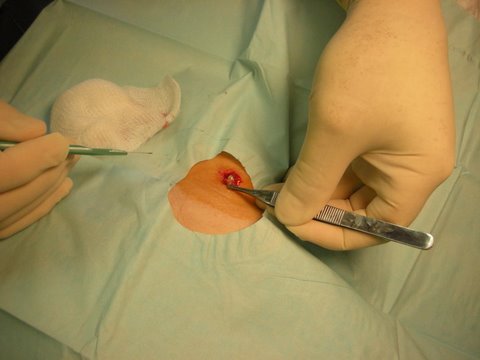
Pearly appearance of small sebaceous cyst
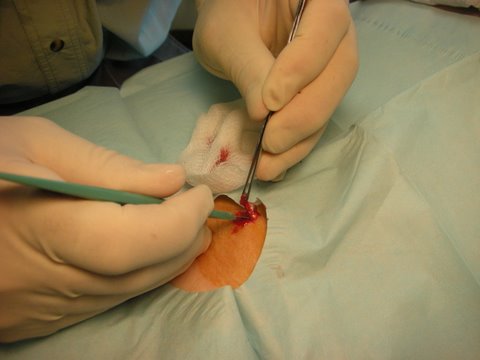
Excision of sebaceous cyst
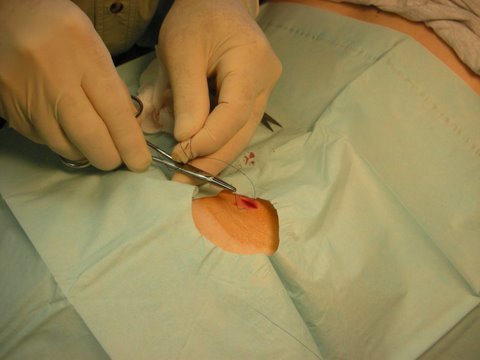
Wound closure with simple sutures
May 19th, 2009 by RamonaBatesMD in Better Health Network, Health Tips
No Comments »

Being a plastic surgeon, I have a great interest in the skin and no I don’t see or treat much dermatitis as the primary physician. Patients do occasionally ask me about patches/rashes they have. It’s always nice to be up on the topic and to know when it’s important to make sure they see a dermatologist.
The article listed below is a nice, simple review of conditions that fall into the eczema /dermatitis categories. The article discusses atopic dermatitis (AD), nummular (coin-shaped) eczema, contact dermatitis, and stasis dermatitis. It is not a deep article on the subject, but did include some nice reminders and tips.
Allergic dermatitis is not uncommon in patients with chronic wounds. One study documented more than 51% of leg ulcer patients acquire contact allergic dermatitis to local dressings and other topical treatment. This is important to any of us who treat wounds, acute or chronic. Sometimes the wound fails to heal due to this.
There is a nice table which lists the common allergens in patients with chronic wounds. If your chronic wound patient has a contact allergy to these products, it can certainly complicate their wound healing.
-
lanolin (common in moisturizing creams and ointments)
-
perfumes/fragrances
-
cetylsterol alcohol (used as an emulsifier, stabilizer, and preservative in creams, ointments, and paste bandages)
-
preservatives: quaternium 15, parabens, chlorocresol (all are used to prevent bacterial contamination in creams, but are not in ointments)
-
rosin (colophony) — a component of some adhesive tapes, bandages, or dressings
-
rubber / latex
The key to treatment and prevention of future exacerbations is identification of any provocative factors so that they may be avoided as there is no absolute cure for dermatitis. Here is a summary of tips the article gives:
Laundry and Clothing Suggestions
-
Avoid wearing wool or nylon next to their skin as they may exacerbate itch. Choose materials made of cotton or corduroy which are softer.
-
Rather than use fabric softeners and bleach, which may be irritating to the skin, add a white vinegar rinse in the washing machine rinse cycle cup/dispenser to remove excess alkaline detergent.
Moisturizers
-
Keep water exposure to a minimum.
-
Use humectants or lubricants regularly to replenish skin moisture. Apply these agents immediately after bathing while the skin is damp.
-
For severe hand eczema, cotton gloves may be worn at night to augment the moisturizing effect of humectants and other topical treatments.
Topical Steroids
-
Topical steroids continue to be the mainstay therapy for treating dermatitis.
-
Topical steroid creams can be kept in the refrigerator or combined with 0.5% to 1% of menthol (camphor and phenol are alternatives) to give a cooling effect. This often helps.
-
Treat the dermatitis with a topical steroid when the skin is red and inflamed. Tapering the topical steroid use by alternating with moisturizers as the dermatitis resolves.
-
Remember that percutaneous absorption of topical steroids is greatest on the face and in body folds. They suggest only weak or moderate preparations be used in these areas.
-
Moderate to potent topical steroids should be used on the trunk and the extremities.
-
The palms and soles are low-absorption areas, so may require very potent topical steroids
REFERENCE
The ABCs of Skin Care for Wound Care Clinicians: Dermatitis and Eczema; Advances in Skin & Wound Care: May 2009, Vol 22, Issue 5, pp 230-236; Woo, Kevin Y. RN, MSc, PhD, ACNP, GNC(C), FAPWCA; Sibbald, R. Gary BSc, MD, MEd, FRCPC (Med, Derm), ABIM DABD, FAPWCA (doi:10.1097/01.ASW.0000350837.17691.7f)
*This blog post was originally published at Suture for a Living*
May 17th, 2009 by Dr. Val Jones in True Stories
3 Comments »
Tragically, land mines injure between 15,000 to 20,000 people each year. Some civilians see a metal object sticking out of the ground and attempt to pick it up and inspect it – the result is often loss of both hands and eyes.
The goal of rehabilitation after trauma is to restore as much independence as possible to patients. With loss of vision and no hands, self care, feeding, and donning/doffing arm prostheses can be very challenging. There is a procedure, known as the Krukenberg operation (named after Hermann Von Krukenberg, who first described it in 1917), that allows the forearm bones to be separated, using the muscle rotators that exist between them to create a pincer grasp. This procedure is not uncommonly used in India and Pakistan and does indeed return some degree of functional use to the arms.
At a recent Physical Medicine and Rehabilitation conference, this photograph was used to illustrate arm function after the Krukenberg operation.
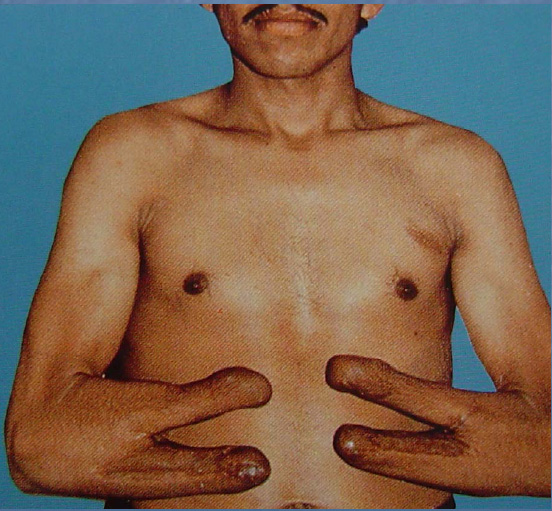
Photo Credit: Dr. Heikki Uustal
It certainly presents a conundrum – should function trump aesthetics in all cases?
I’m not sure that I’d want this procedure, even if I lost my vision and both hands.
Would you?
May 12th, 2009 by RamonaBatesMD in Better Health Network
No Comments »

The U.S. Food and Drug Administration recently announced that safety label changes including a boxed warning and a Risk Evaluation and Mitigation Strategy (REMS) will now be required for all botulinum toxin products. The agency took the action because of two main reasons. The first one is the potential for serious risks that may occur from the spread of the botulinum toxin beyond the injection site. The second reason is associated with the lack of interchangeability among the three licensed botulinum toxin products.
When the botulinum toxin spreads beyond the area of injection, symptoms similar to botulism may occur. These symptoms include unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids.
This “spreading effect” has been reported in both children and adults. It has been reported most often in children with cerebral palsy being treated with the products for muscle spasticity. Treatment of muscle spasticity is an off-label use of the drug. The “spreading effect” has been reported in patients being treated for both approved and unapproved uses.
Botulinum toxin products include:
-
Botox and Botox Cosmetic (botulinum toxin type A), marketed by Allergan
-
Myobloc (botulinum toxin type B), marketed by Solstice Neurosciences
-
Dysport (abobotulinumtoxinA), a new FDA-approved product marketed by Ipsen Biopharm Ltd.
All are approved by the FDA for the treatment of cervical dystonia. Botox Cosmetic and Dysport are approved by the FDA for treatment of glabellar frown lines. Botox is approved for the treatment of severe underarm sweating (primary axillary hyperhidrosis), crossed eyes (strabismus), and abnormal tics and twitches of the eyelids (blepharospasm). All other uses are considered off-label.
The FDA has not identified any definitive serious adverse event reports of a distant spread of toxin effect producing symptoms consistent with botulism when these products are used in accordance with the approved label.
It is important for those of us who use botulinum toxins to remember that the dosage strength between the products is not the same. One unit of Botox is not equal in strength (potency) as one unit of Myobloc or Dysport.
BOTOX® Cosmetic Insert (pdf)
Dilution Technique: Using a 21-gauge needle and an appropriately sized syringe draw up a total of 2.5 mL/100 Unit vial or 1.25 mL/50 Unit vial of 0.9% sterile saline without a preservative…….
Injection Technique: Glabellar
Using a 30-gauge needle, inject a dose of 0.1 mL into each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 20 Units. Typically the initial doses of reconstituted BOTOX® Cosmetic induce chemical denervation of the injected muscles one to two days after injection, increasing in intensity during the first week.
MYOBLOC® Insert (pdf)
The recommended initial dose of MYOBLOC® for patients with a prior history of tolerating botulinum toxin injections is 2500 to 5000 U divided among affected muscles. Patients without a prior history of tolerating botulinum toxin injections should receive a lower initial dose. The duration of effect in patients responding to MYOBLOC® treatment has been observed in studies to be between 12 and 16 weeks at doses of 5000 U or 10,000 U.
Dysport® Insert
The units of Dysport are specific to the preparation and are not interchangeable with other preparations of botulinum toxin.
Glabellar lines Dosage
The dosage is dependant on the severity of the lines and the specific muscle being treated.
For the corrugator and procerus muscles 40 to 60 units divided between injection sites as follows:
8 to 12 units in each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 60 units
Improvement of severity of glabellar lines generally occurs within 72 hours after treatment and persists for 3 to 6 months.
It is important for us to educate patients and their caregivers of the potential adverse effects. Some of these effects have been reported as early as several hours and as late as several weeks after treatment. Patients should seek immediate medical attention if they develop any of these symptoms — unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids.
Adverse events may be reported by health care professionals and/or consumers to the FDA’s MedWatch Adverse Event Reporting program by four ways:
REFERENCES
FDA News (April 30, 2009)
BOTOX® Injections; eMedicine Article, Sept 25, 2008; Robert A Hauser, MD, MBA, Mervat Wahba, MD, Theresa McClain, MSN, ARNP
*This blog post was originally published at Suture for a Living*















