November 24th, 2011 by AndrewSchorr in Opinion
No Comments »

First, to our U.S. visitors – Happy Thanksgiving! To our friends around the world, we send all the best to you too!
This tends to be a time of reflection so I am heavy into that right now. First, as a 15-year-leukemia survivor, I am thankful every day I can live a full life. And, as the founder of Patient Power, I am thankful to you for visiting our site, listening to and watching our programs, and for telling us that, for the most part, we are making a positive difference in your life or that of a loved one as you seek better health. Quite frankly, I am thrilled when I read the comments you make in our surveys and your feedback propels all of us to do more. Read more »
*This blog post was originally published at Andrew's Blog*
November 24th, 2011 by PJSkerrett in News
No Comments »

Last week, the FDA revoked its 2008 approval of the drug Avastin to treat breast cancer, concluding that the drug does little to help women with breast cancer while putting them at risk for potentially life-threatening side effects. Avastin will remain on the market (and so be potentially available to women with breast cancer) because it has also been approved to treat other types of cancer.
In a statement, FDA Commissioner Margaret A. Hamburg said this:
FDA recognizes how hard it is for patients and their families to cope with metastatic breast cancer and how great a need there is for more effective treatments. But patients must have confidence that the drugs they take are both safe and effective for their intended use. After reviewing the available studies it is clear that women who take Avastin for metastatic breast cancer risk potentially life-threatening side effects without proof that the use of Avastin will provide a benefit, in terms of delay in tumor growth, that would justify those risks. Nor is there evidence that use of Avastin will either help them live longer or improve their quality of life.
*This blog post was originally published at Harvard Health Blog*
October 19th, 2011 by AndrewSchorr in Health Policy, Interviews
No Comments »

If you’ve read my blogs for a while, or look up some past blogs, you’ll see I have been frustrated at times with the FDA. Yes, they have a tough job protecting us from medical products that are unsafe and/or ineffective. But when it comes to cancer, where we have few “homerun” therapies, I wish they were a bit more liberal. A “bunt single” might be good enough. You may have read how I have been critical of Dr. Rick Pazdur, the FDA leader for oncology drug approval. Some desperate patients and family members have referred to him as “Dr. No.”
Just the other day I interviewed a respected breast cancer survivor and patient advocate who has high respect for Dr. Pazdur. Musa Mayer of New York City is a 22-year breast cancer survivor and author of three books about breast cancer. She’s devoted her life to educating other patients about cancer and also playing a role in public policy. She has become a favorite patient representative on FDA cancer advisory boards and regularly weighs in when breast cancer drugs are being considered.
In my interview with Musa, she explained Read more »
*This blog post was originally published at Andrew's Blog*
October 18th, 2011 by RyanDuBosar in Research
No Comments »

Oropharyngeal cancers caused by the human papillomavirus (HPV) are on the rise in the United States since 1984, as changes in sexual habits further the virus’ spread. But the focus of the HPV vaccine will remain on preventing genital warts and cervical cancer.
Reuters reported one clinician’s opinion that throat cancer linked to HPV will become the dominant cause of the disease, ahead of tobacco use.
To study the issue, researchers determined HPV-positive status among 271 of all 5,755 oropharyngeal cancers collected by the three population-based cancer registries in Hawaii, Iowa and Los Angeles from the Surveillance, Epidemiology, and End Results (SEER) program from 1984 to 2004. Prevalence trends across four calendar periods were estimated by using logistic regression. The study appeared online Oct. 3 in the Journal of Clinical Oncology.
HPV prevalence Read more »
*This blog post was originally published at ACP Internist*
October 16th, 2011 by Elaine Schattner, M.D. in Research
No Comments »

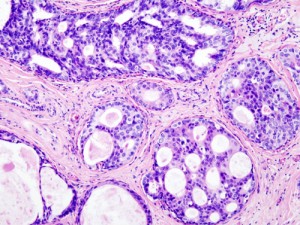
Ductal Carcinoma in Situ (DCIS) in the breast, histopathology w/ hematoxylin & eosin stain, Wiki-Commons image
More, a magazine “for women of style & substance,” has an unusually thorough, now-available article by Nancy F. Smith in its September issue on A Breast Cancer You May Not Need to Treat.
The article’s subject is DCIS (Ductal Carcinoma in Situ). This non-invasive, “Stage 0” malignancy of the breast has shot up in reported incidence over the past two decades. It’s one of the so-called slow-growing tumors detected by mammography; a woman can have DCIS without a mass or invasive breast cancer.
While some people with this diagnosis choose to have surgery, radiation or hormonal treatments, others opt for a watchful waiting strategy. The article quotes several physicians, including oncologists, who consider Read more »
*This blog post was originally published at Medical Lessons*















