July 10th, 2012 by Dr. Val Jones in Health Tips, News
No Comments »
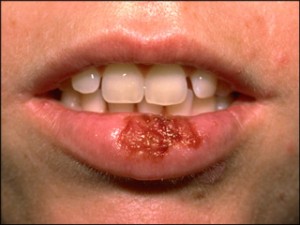 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
In a recent survey (sponsored by Abreva), 66% of respondents said they believed that “look-alike” medications contained the same active ingredients as the brands that they were copying, and 93% said they purchased look-alikes solely because they were less expensive.
Unfortunately, you get what you pay for in this case. Even though the FDA may send out warning letters to manufacturers of look-alike products who print false healing claims on their packaging, those products often remain on store shelves. The manufacturer of Abreva (Glaxo Smith Kline) notes in a press release:
Several “look-alike” cold sore treatments tout healing claims, but contain the ingredient Benzalkonium Chloride instead of docosonal. The FDA recently issued a warning letter to a marketer and distributor of a product containing benzalkonium chloride that is making the claim on its product’s label that it heals cold sores. The FDA found that the active ingredient, benzalkonium chloride, is not indicated as a cold sore treatment and may not make cold sore healing claims because there is no scientific evidence to support claims that it heals cold sores.
Evidence suggests that Abreva shortens the duration of cold sores by about 18 hours, and may slightly reduce the risk of outbreak if the medicine is used at the first sign of pain or tingling. Whether that’s worth the price of the treatment is up to the consumer, but choosing a cheaper product with a different active ingredient is likely to be a waste of money.
The Abreva case serves as a reminder to check the active ingredients in your OTC medicines before purchasing what seems to be a cheaper, equivalent medicine. While generics are often a smart way to save money on effective medicines, look-alike medicines can sometimes be hiding fake cures in a convincing little package. Let the buyer beware!
January 6th, 2012 by RyanDuBosar in News
No Comments »


Cephalosporins will be used in livestock only for very specific exceptions, after years of debate about the role of antibiotic resistance in farming and how it leads to new strains of microbes with the potential to shift into humans.
The FDA took this step to preserve the effectiveness of cephalosporin drugs for treating disease in humans, the agency announced in a press release.
In 2008, the FDA issued and then revoked an order that prohibited cephalosporins in food-producing animals with no exceptions. Three years later, the agency’s ban includes several exceptions:
–It doesn’t limit cephapirin, which the FDA doesn’t think contributes to antimicrobial resistance;
–Veterinarians will still be able to Read more »
*This blog post was originally published at ACP Internist*
January 5th, 2012 by DavidHarlow in Health Policy, Opinion
No Comments »

There are at least two conversations going on in the health care marketplace today, each focused on one of two key questions. One is: How can we achieve the Triple Aim? The other is: Why do they get to do that? (It’s not fair! I want more!)
Until we stop asking the second question, we can’t answer the first question. Why? Because all too often the answer to the second question is the equivalent of: It’s OK, Timmy, I’ll buy you TWO lollipops; pick whichever ones you want.
It’s the tragedy of the commons, transposed to the health care marketplace.
Recent cases in point:
- Avastin
- Tufts Medical Center – Blue Cross Blue Shield of Massachusetts grudge match
- Mammography and PSA guidelines
1. Avastin. Late last year, Read more »
*This blog post was originally published at HealthBlawg :: David Harlow's Health Care Law Blog*
December 22nd, 2011 by John Di Saia, M.D. in News, Opinion
No Comments »

The U.S. Food and Drug Administration today announced that it has taken action against eight California surgical centers and the marketing firm 1-800-GET-THIN LLC, for misleading advertising of the Lap-Band, an FDA-approved device used for weight loss in obese adults. The FDA issued Warning Letters to Bakersfield Surgery Institute Inc.; Beverly Hills Surgery Center; Palmdale Ambulatory Center; Valley Surgical Center; Top Surgeons LLC; Valencia Ambulatory Center LLC; Cosmopolitan Plastic & Reconstructive Surgery; San Diego Ambulatory Center LLC; and to 1-800-GET-THIN because Lap-Band is a restricted medical device that is misbranded as a result of misleading advertising by these groups. In the letters, the FDA warns that billboards and advertising inserts used by recipients of the Warning Letters to promote the Lap-Band procedure fail to provide required risk information, including warnings, precautions, possible side effects and contraindications. The FDA also is concerned that the font size of information related to risks on the advertising inserts is too small to be read by consumers.
Source: fda.gov/NewsEvents/Newsroom/PressAnnouncements
/ucm283455.htm#.TueG3YY1aZY.facebook
We have blogged on 1-800-Get-Thin and Lap-band surgery in general before. Read more »
*This blog post was originally published at Truth in Cosmetic Surgery*
December 20th, 2011 by DrWes in Opinion
No Comments »

“How are you feeling, Ms. Jones?”
“Fine.”
“Have you been more short of breath lately?”
“Not really, just when I exercise.”
“How much exercise?”
“I dunno. But after I go to the mailbox and walk back up to the house, I’ve got to stop now where before I didn’t.”
Exertional dyspnea. It conjures up a large differential of potential cardiovascular or pulmonary causes. And as the above commonly-encountered doctor-patient conversation demonstrates, the problem is a dynamic one: at rest things are often fine, on exertion or with recumbency less so.
Now imagine that the doctor then sees elevated neck veins, hears rales in the lower lung fields, and sees swollen ankles on their patient. Heart failure, right? Read more »
*This blog post was originally published at Dr. Wes*
 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).














