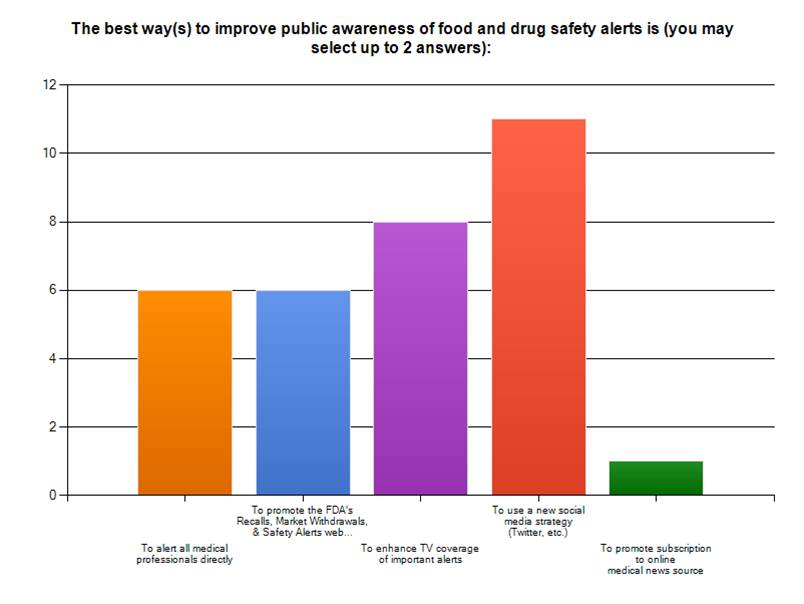
June 29th, 2009 by admin in Better Health Network, Health Policy
No Comments »
Much has been made of the recent pressure on the FDA to create a pathway for follow-on biologics. On June 9, 2009, Rep. Henry Waxman, chair of the Energy and Commerce committee, sent a letter to President Obama imploring him to approve a pathway for generic biologics: “I urge the administration to consider what steps can be taken under existing authority to prepare and even begin to use a pathway for generic biologics.”
Six days later, President Obama, in his June 15, 2009 speech to the AMA, followed Waxman’s lead, asking the FDA and industry “to introduce generic biologic drugs into the marketplace.” He continued: “These are drugs used to treat illnesses like anemia. But right now, there is no pathway at the FDA for approving generic versions of these drugs. Creating such a pathway will save us billions of dollars.”
Is this true? Are follow-on biologics, biosimilars, or generic biologics (all names for the same concept) truly the path to healthcare savings? And what are they, anyway? To clear up the confusion, this post aims to explain what biologics are, what generics are, and what the challenges are in the creation of an approval pathway for generic biologics.
What are biologics? How do they differ from traditional small-molecule therapies?
When you think of a drug, you probably think of a pill like Tylenol or Lipitor. You may not be as familiar with biologics, which are attracting a great deal of attention from policymakers and media. Biologics represent a different set of drugs, distinguished by their size, their informational and manufacturing complexity, and their therapeutic advantages.
If you were to look at most drugs under a microsope, you would find that they are actually rather small relative to a typical biologic. Acetaminophen, which is the active ingredient in Tylenol, weighs 150 daltons (a dalton is a unit of atomic mass). Enbrel, on the other hand, which is a top-selling biologic indicated for several autoimmune diseases, weighs 150,000 daltons. To put that thousand-fold difference in visual terms, if Tylenol weighed as much as your mountain bike, then Enbrel would weigh as much as an unfueled 12-passenger private jet. Hence you will often hear people in the pharmaceutical industry refer to “small molecule” drugs and “large molecule” drugs — because these are two truly distinct classes.
Biologics are often designed to closely mimic the body’s own specific natural processes. Because of this higher specificity, biologics tend to bind to drug targets in the body more precisely than do traditional drugs, which may bind to other unintended targets in the body, placing the patient at greater risk of side effects.
On top of it all, there is currently no defined pathway at the FDA for companies to develop generic versions of biologics, so biologic manufacturers retain data exclusivity over their products. Not surprisingly, the therapeutic and market advantages of biologics have caused pharmaceutical companies to focus their efforts on developing or acquiring biologics over small-molecule drugs. In fact, according to a recent forecast, by 2014, seven of the top ten drugs in America will be biologics, including every single one of the top six.
Unfortunately, while biologic therapies provide a great deal of therapeutic benefits, they are also extremely challenging for biopharmaceutical companies to develop and manufacture, because they are composed of entire proteins, carefully grown through recombinant DNA methods which are newer and less practiced than traditional drug chemistry methodologies. A traditional drug is usually derived through a set of chemical reactions. It’s a lot like following a recipe. Synthesizing a biologic, however, is a bit more like cloning a cat. In order to synthesize a biologic, host mammalian cells, usually Chinese hamster ovary cells, must be implanted with a gene that codes for the desired drug. The host cells are maintained in a special bioreactor that is designed to keep the cells alive as they translate, synthesize and excrete the drug. The broth of cells must then be harvested from the cells, modified, purified, and tested. The solution is finally packaged into vials or pre-filled syringes for distribution.
This manufacturing process is unusually challenging to reproduce consistently, even within a company. For example, Johnson & Johnson manufactures epoetin alfa, an anemia therapy, under the name EPREX in Europe. In the late 1990s, J&J changed its manufacturing process for EPREX at the request of the EMEA (the European version of the FDA). The process change caused some patients to develop pure red blood cell aplasia, a serious adverse reaction. Rather than receiving the benefit of the anemia therapy, these patients actually lost their ability to make red blood cells because they produced an antibody (triggered by the faulty EPREX) that inactivated both the EPREX and the body’s natural protein that is essential for red blood cell production. J&J eventually determined the cause of the adverse reaction and corrected it, but only after a lengthy and expensive investigation.
Because of the intense development and manufacturing process, biologics are also significantly more expensive than traditional therapies. Herceptin, an effective treatment for some forms of breast cancer, can cost as much as $48,000 for one year’s worth of treatment. It’s important to keep in mind, however, that virtually every drug company provides programs to help underinsured or uninsured patients get financial assistance in the form of co-pay cards, co-pay grants, or free drug programs. Simply contact the drug manufacturer.
Why have biologics gotten so much attention from healthcare reformers such as Rep. Waxman and President Obama?
The high cost of biologic therapies has attracted attention from both private payers, such as Aetna and UnitedHealthcare, and public payers, such as Medicare, Medicaid, and state health insurance programs. While payers agree that the therapeutic benefits of these treatments are important, they are still anxious to limit their exposure to the high price tags. Most insurers require several other therapeutic steps before allowing a physician to prescribe a biologic therapy.
Wait, what exactly is a generic? And what’s all this talk of bioequivalence?
The Hatch-Waxman Act of 1984 established a pathway for generic versions of small-molecule drugs to be offered to the public. Once the patent ends on a drug, generic drug makers may manufacture and sell the same drug without repeating the research, expensive clinical trials, or marketing efforts conducted by the original patent holder. These savings allow generic manufacturers to sell their versions for a lot less.
In order to gain approval, the maker of the generic must still show bioequivalence to the original drug, called the “reference listed drug” in the generic drug maker’s application. In a bioequivalence study, the reference listed drug is administered to one group of healthy volunteers, and the generic is administered to a second group. The blood concentrations of the active ingredients are measured over time and graphed. If the generic drug’s graph lies between 80% and 125% of the graph of the reference listed drug, then the two are deemed bioequivalent, and the generic drug is approved. Once approved for the market, it may be sold and independently substituted by a pharmacist for the branded medication without telling the physician, assuming the doctor has not expressly forbidden generic substitution. This last permission is referred to as the “interchangeability” of the drug.
Why can’t Congress just duplicate the same approval process used for generic small-molecule drugs?
In theory, Congress could. In practice, however, there are several technical hurdles that remain to be cleared. As discussed above, the processes used to create biologic therapies are extremely sensitive to manufacturing changes, as in the EPREX case. If a generic biologic manufacturer develops its own process, there is a good chance that the quality of the product would differ from that of the reference listed drug. Furthermore, no one has yet confidently measured bioequivalence for a biologic.
Frank Torti, Chief Scientist of the FDA, summarized these issues very well in a September 2008 response to a Congressional inquiry about follow-on biologics:
Because of the variability and complexity of protein molecules, current limitations of analytical methods, and the difficulties in manufacturing a consistent product, it is unlikely that, for most proteins, a manufacturer of a follow-on protein product could demonstrate that its product is identical to an already approved product. Technology is not yet sufficiently advanced to allow this type of comparison for more complex protein products.
All is not lost, though. The FDA could still create a pathway for generic biologic manufacturers to develop “biosimilars,” which are products that are intended to be close to a reference listed drug but cannot be shown to be the same. Because they are not the same, biosimilar manufacturers would likely have to conduct clinical trials to show that their version is safe and effective for human use, and can be manufactured consistently.
What are the realistic cost savings?
Because of the added cost of clinical development, testing, and marketing of a biosimilar product on top of the difficult manufacturing process, and competition, generic biologic pricing is more likely to resemble brand-to-brand biologic competition than the generic-to-brand competition seen in the small-molecule drug marketplace. Therefore, it’s not yet clear how much more affordable a FOB would be for health insurers. Without being able to show that the products are truly identical and therefore interchangeable, physicians are also likely to be reluctant to try what is essentially a “new” drug that does not truly share the established track record of the original drug. Payers and patients may be excited about the lower cost but skeptical of potential safety issues. As a result of these factors, generic biologic manufacturers may ultimately fail to capture enough business to make up for the upfront expenses of clinical testing, as well as the ongoing manufacturing and marketing expenses.
The Federal Trade Commission recently published a report that studied and called out these limitations. The consequences of the market dynamics imply that only two or three companies with large biologics manufacturing capabilities will even bother to get involved in this field, because only those companies will already have the plants and people to compete effectively. Ironically, the FOB manufacturer for a given reference drug will probably be other biologics innovators who already have the manufacturing capabilities but don’t normally compete in that particular market.
What would be some of the other implementation challenges for the government?
For one, CMS would need to decide how to bill and code for the new products, a subtle referendum on how identical the biosimilars will really be. If they give the generic versions the same codes as the originals, interchangeability is easier and the cost savings are more likely to materialize. On the other hand, it’s important for both the FDA and CMS to track adverse events with these new products (an activity known as “pharmacovigilance”), which is easier to do if the codes are different.
Where does the policy debate stand? What are the Eshoo and Waxman proposals?
The current Waxman bill is remarkably similar to the Hatch-Waxman Act of 1984, which was originally designed for small-molecule drugs. It would not require any new clinical trials for generic biologics provided that the generic had a “highly similar molecular structures,” and allows a case-by-case determination on whether or not safety and efficacy data would be required before pharmacies could substitute generics for reference biologics without telling the physician, but the default would be to allow substitution on approval. The Waxman bill allows for five to nine years of data exclusivity for the original patent holder.
The current Eshoo bill would require clinical trials comparing the generic biologic to the reference biologic, unless the FDA waived them. Rather than automatically granting interchangeability upon approval, the FDA would have to publish guidance with data that describe the criteria required for interchangeability. The bill also recognizes the greater challenge in developing biologics by allowing for twelve to fourteen years of data exclusivity.
Can the healthcare system really save billions of dollars with biosimilars?
President Obama’s speech to the AMA suggested that billions of dollars would be saved by the creation of a biosimilars approval pathway. Several others to study the issue have cited fairly conservative numbers. Avalere Health put the federal savings at $3.6 billion over a ten-year period, while the Congressional Budget Office says $6.6 billion. Avalere’s model assumes moderate discounting, several entrants, slow uptake of biosimilars, and a ten-year data exclusivity period. The CBO report scores a bill that resembles the Eshoo option described above, but doesn’t account for some of the market dynamics discussed above and in the FTC report.
Finally, to keep pharmaceutical costs in perspective, policymakers should remember that prescription drugs currently make up only 10% of healthcare costs, while physician services make up 21% and hospital care makes up 31%. The CBO estimate also predicts that follow-on biologics would save $25 billion on national biologics expenditures over ten years. Even if correct, those savings still make up one-half of one percent of all national spending on prescription drugs, which is itself one-tenth of all healthcare spending in the United States.
Which option makes more sense?
Overall, the Eshoo bill appears to do the best job of reflecting the current technical challenges particular to biologic therapies. The need for clinical trials to insure the safety, efficacy and quality of FOBs ought to be non-negotiable. However, given the high cost of becoming a FOB manufacturer, and the small number of likely entrants, the optimal length of data exclusivity is a good open question. Henry Grabowski of Duke University studied the issue and concluded that an ideal breakeven point is 12.9 and 16.2 years, also suggesting that the Eshoo option is the most likely to drive economic growth. The European Union currently allows for biosimilars and permits ten to eleven years of data exclusivity. Let’s hope that policymakers work hard to thoughtfully strike the right balance that maintains both a stream of innovative therapies from scientific pioneers and a structure that wisely manages costs for payors.
Author’s bio:
Jonathan Sheffi is a summer intern in the FDA Office of Biotechnology Products. Before the FDA, Jonathan worked for Amgen, first as a marketing analyst and then as a biopharmaceutical sales rep. He will start at Harvard Business School in the fall of 2009, and is seeking an internship opportunity for the summer of 2010. Follow Jonathan’s blog at http://jonathansheffi.com/ and on Twitter at @sheffi.
Acknowledgments:
Thank you to Val Jones (Better Health), Niko Karvounis (The Century Foundation), and Kimberly Barr (UnitedHealthcare) for their comments and suggestions.
Disclosures:
All included information has been derived only from publicly available sources. This blog post reflects the author’s personal opinions and do not represent the opinion of any other organization or individual.
June 22nd, 2009 by Jonathan Foulds, Ph.D. in Better Health Network, Health Policy
No Comments »

On Friday 12th June, the US Senate voted in favor of a bill that gives the Food and Drug Administration the power to regulate tobacco products. President Obama (who as a Senator was a sponsor of the bill, as was John McCain) has indicated he will sign the bill into law.
Public health advocates have been fighting for FDA regulation of tobacco for over 15 years. However, not every public health advocate has supported this particular piece of legislation, and the fact that Philip Morris Tobacco Company supported it caused many to have doubts. But now that the bill is finally going to be law, what will it mean? As a guide to this, I’d recommend that you read the slides from a paper by Mitch Zeller at the recent Virginia Youth Tobacco Conference. Much of this post is adapted from his slides. These outline the key parts of the bill and what they mean in some detail and are worth a careful read, (Mitch Zeller is the former Associate Commissioner and Director of the Office of Tobacco Programs at the Food and Drug Administration). Download his slides here.
The bill does not require FDA to regulate tobacco exactly the same way it does pharmaceuticals (drugs and devices), partly because it would be impossible for any tobacco product to demonstrate that it is safe and effective for its purpose. So instead the legislation creates a new, parallel set of rules and procedures just for tobacco, but using much of the same regulatory framework that was created for drugs. The new standard around which the tobacco bill is based is THE PROTECTION OF PUBLIC HEALTH.
-Section 904 mandates that FDA will receive brand-specific information on ingredients, nicotine delivery, and any smoke constituent FDA identifies as harmful or potentially harmful
– Companies must also provide FDA with all documents developed after the bill is enacted related to health, toxicological, behavioral, or physiologic effects of current or future products.
– FDA also has the right to request any such industry document produced prior to the enactment of this law.
– FDA can issue performance standards to prohibit or limit the allowable levels of substances in a finished tobacco product. FDA is granted this power in section 907. Products that don’t comply with the levels established in product standards can’t be sold.
– Nicotine cannot be banned but it can be reduced to very low levels.
– FDA needs to have supporting science to back up any standards it requires, or any ingredients it wants banned.
Clearly the tobacco product standards are going to be a key part of regulation of tobacco. Here’s what the bill says on these: Read more »
This post, FDA Regulation Of Tobacco: What Does It Mean?, was originally published on
Healthine.com by Jonathan Foulds, Ph.D..
May 26th, 2009 by admin in Better Health Network, Quackery Exposed
No Comments »

By Dr. John Snyder of the Science Based Medicine Blog
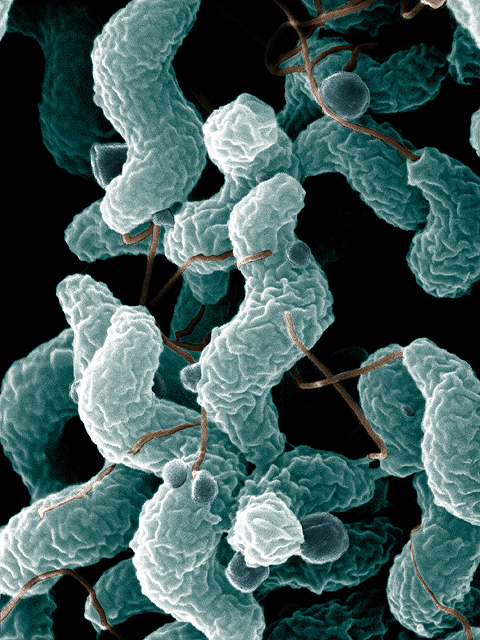
I recently saw a 14 year old girl in my office with a 2 day history of severe abdominal cramps, bloody diarrhea, and fever. Her mother had similar symptoms as did several other members of her household and some family friends. After considerable discomfort, everyone recovered within a few days. The child’s stool culture grew a bacterium called Campylobacter.
Campylobacter is a nasty little pathogen which causes illness like that seen in my patient, but can also cause more severe disease. It is found commonly in both wild and domestic animals. But where did all these friends and family members get their campylobacter infections? Why, from their friendly farmer, of course!
My patient’s family and friends had taken a weekend pilgrimage to a family-run farm in Buck’s County, Pennsylvania. They saw farm animals and a working farm. And they all drank raw milk. Why raw milk? Because, as they were told and led to believe, raw milk is better. Better tasting and better for you.
In 1862, the french chemist Louis Pasteur discovered that heating wine to just below its boiling point could prevent spoilage. Now this process (known as pasteurization) is used to reduce the number of dangerous infectious organisms in many products, prolonging shelf life and preventing serious illness and death. But a growing trend toward more natural foods and eating habits has led to an interest in unpasteurized foods such as milk and cheese. In addition to superior taste, many claim that raw milk products provide health benefits not found in the adulterated versions. Claims made about the “good bacteria” (like Lactobacillus) conquering the “bad” bacteria (like Campylobacter, Salmonella, and E. coli) in raw milk are pure fantasy. Some even claim that the drinking of mass-produced, pasteurized milk has resulted in an increase in allergies, heart disease, cancer, and a variety of other diseases. Again, this lacks any scientific credibility.
With this growing interest in unpasteurized dairy products has come an increase in the rate of food-born infections. The federal government developed the Grade ‘A’ Pasteurized Milk Ordinance in 1924, providing a set of guidelines for the safe processing and handling of milk products. Although all 50 states have voluntarily adopted these guidelines, the FDA has no oversight jurisdiction. It is up to individual states to determine their own safety protocols and enforcement strategies. While selling raw milk is currently illegal in 26 states, those with a will have found a way to skirt the law to get their fix of the real deal.
My patient was a victim of a recent outbreak in Pennsylvania, but similar outbreaks of infectious disease due to unpasteurized milk products are a recurring headache for public health officials. Between 1973 and 1993 there was an average of 2.3 milk born disease outbreaks per year. That number increased to 5.2 per year between 1993 and 2006. Whatever the numbers are, there is no question that the increasing consumption of raw milk is a genuine threat to public health.
The health claims made for raw milk, and against its pasteurized cousin, are being heavily pushed by a small but passionate contingent one might refer to as “food guardians.” These are people who espouse a return to the good old agrarian days of wholesome, farm-raised foods, free from man-made chemicals and mass-market processing. Some of these ideals are highly respectable and healthful responses to the ways in which society has dealt with the need to push products to a mass market at profit. For example, the use of pesticides, animal hormones and antibiotics, and farm run-off can have deleterious environmental and human health consequences. However, many of the health claims that are made about products like raw milk are not supported by scientific evidence, and lack scientific plausibility. Despite this lack of evidence, however, the allure of raw milk products is clearly on the rise.
Beyond the obvious public health consequences of this trend lies the problem of an increasing public credulousness when it comes to pseudoscientific claims. This is similar to the trend we are seeing regarding concerns about the dangers of vaccines and excessive fears concerning certain potential environmental hazards.
Unscientific and outright fraudulent claims about the health benefits (as well as the hidden dangers) of a variety of foods is on the rise. And bogus or unsupported nutrition claims are big business. From the immune boosting and weight loss powers of the acai berry, to the cancer protective effects of vitamins, nutrition pseudoscience is all the rage. While raw milk will never have quite the celebrity cache of these “super foods”, it is promoted with the same lofty yet empty claims, and provides the added bonus of infectious diarrhea.
On a recent visit to a local high-end wine shop, I came face-to-face with the ease with which people fall prey to the marketing of food pseudoscience. A woman was examining a bottle of wine when the store keeper approached to offer help. She told her a little about the wine and then said, “And all of their wines are biodynamic.” To this, the shopper exclaimed “Oh wow, that’s great.” She bought the wine, likely without having a clue what the term “biodynamic” even means. Biodynamic farming is a mixture of Gaia-like principles (the earth is a living organism) and organic practices, with a smattering of mysticism, alchemy, and astrology. In essence, a smorgasbord of pseudoscientific farming practices perfect for the current culture of armchair environmentalism and the new found heal-thy-self mantra of the well-to-do. While the motivating factors and socioeconomic status may differ between those drinking biodynamic wine and those drinking raw milk, both are relying on false beliefs and unsupported claims in making their choices to consume these products.
As a lover of cheese, I appreciate that there are those whose refined palates favor the delicacy of unpasteurized, aged cheeses so prevalent in other countries. But to stretch this taste preference to include health benefits unsupported by science and even common sense is not just misguided, it can be dangerous. Dangerous because it increases the risk of infectious disease, but also because it perpetuates a credulous perspective that adds to the ongoing erosion of our appreciation and acceptance of science.
*This blog post was originally published at Science-Based Medicine*