September 7th, 2011 by RamonaBatesMD in News, Opinion
No Comments »


It’s amazing what you will find sorting through more than 20 years of stuff. This picture of 3 implants includes: top — an old McGhan double lumen (silicone gel implant surrounded by a saline implant); bottom left – Dow Corning textured silicone implant; and bottom right – Dow Corning smooth silicone implant. Dow Corning has not made breast implants since approximately 1992.
Last week the FDA met to discuss and make recommendations on postmarketing issues related to silicone gel-filled breast implants. As a condition of placing silicone implants back on the market in 2006, both Mentor and Allergan (McGhan) were supposed to enroll patients in 10-year-long follow up studies on side effects related to implants. The aim was for 80,000 women.
I agree these studies are needed, but it is difficult to get women to return year after year. This is evident in the data presented at the meeting: Read more »
*This blog post was originally published at Suture for a Living*
September 4th, 2011 by Medgadget in News, Research
No Comments »

Though mastectomies are often a necessary and even welcome intervention to save the lives of women suffering from breast cancer, they also may contribute to the overall physical and emotional trauma facing the patients. In order to alleviate some of these problems, surgeons have developed breast reconstruction procedures that usually entail restoring the mound by implanting a silicone sac filled with salt solution (saline) or gel under the skin and pectoral muscles. The traditional process to prepare for implantation of the sac may be long and sometimes painful because it involves weekly bolus saline injections (sometimes up to 22 weeks) in order to create a pocket of sufficient size.
A potential alternative solution is being developed by AirXpanders, a med tech start-up in Palo Alto that focuses on tissue expansion for breast reconstruction following cancer. Their system, known as AeroForm, just recently Read more »
*This blog post was originally published at Medgadget*
September 2nd, 2011 by RyanDuBosar in Research
No Comments »

Severe shortages for life-saving medications have driven a “gray market” in the wholesale drug supply industry, a watchdog group reports.
And the mark-up on gray market drugs is a budget-buster, reports the Institute for Safe Medication Practices, a Philadelphia-based nonprofit organization devoted entirely to medication error prevention and safe medication use. Purchasing agents and pharmacists at 549 hospitals responded to a survey on gray market activities associated with drug shortages.
The report includes chilling anecdotes from the respondents about pressure from physicians and administrators to ensure drugs are available, and drastic price gouging from the gray market suppliers. Price mark-ups of 10 times or more than the contract price were reported by about a third of respondents from critical access hospitals and community hospitals, and more than half of university hospitals. Examples include a box of calcium gluconate that cost $750 instead of the contract price of $50 (1,400% mark-up), and a supply of propofol that cost $25,000 instead of $1,500 (1,567% mark-up). Oh, and there’s exorbitant shipping and handling fees, too. Read more »
*This blog post was originally published at ACP Hospitalist*
August 29th, 2011 by Elaine Schattner, M.D. in News
No Comments »

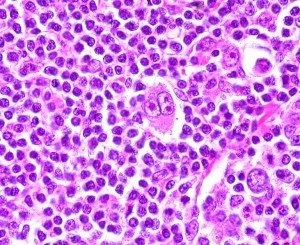 Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).
Recently, the FDA announced its approval, upon accelerated review, of a new drug, Adcetris (brentuximab) for patients with Hodgkin’s lymphoma that has relapsed after bone marrow transplant and for some patients with T-cell anaplastic large cell lymphoma (ALCL).
This interests me for a lot of reasons, among them that I used to work in the field of lymphoma immunology and spent some time in my life studying molecules like CD30, the protein to which the new antibody binds.
First, a mini-primer on the disease and numbers of patients involved: Read more »
*This blog post was originally published at Medical Lessons*
August 15th, 2011 by Medgadget in News
No Comments »

GE Healthcare has received the FDA OK for its Optima CT660 computed tomography (CT) system. The CT660, which is already available in Europe, Latin America and Asia, distinguishes itself by its compact footprint combined with a modular design and low dose imaging. In addition, it is also one of the most energy efficient CT scanners available and has an “environmental design” that eases refurbishment and end-of-life recycling. The scanner itself is scalable from 32 to 128 slices through purchasable options and features automatic table positioning and a color 12-inch integrated gantry display monitor. Read more »
*This blog post was originally published at Medgadget*







 Recently, the FDA
Recently, the FDA 







