July 10th, 2012 by Dr. Val Jones in Health Tips, News
No Comments »
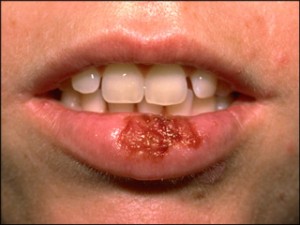 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
In a recent survey (sponsored by Abreva), 66% of respondents said they believed that “look-alike” medications contained the same active ingredients as the brands that they were copying, and 93% said they purchased look-alikes solely because they were less expensive.
Unfortunately, you get what you pay for in this case. Even though the FDA may send out warning letters to manufacturers of look-alike products who print false healing claims on their packaging, those products often remain on store shelves. The manufacturer of Abreva (Glaxo Smith Kline) notes in a press release:
Several “look-alike” cold sore treatments tout healing claims, but contain the ingredient Benzalkonium Chloride instead of docosonal. The FDA recently issued a warning letter to a marketer and distributor of a product containing benzalkonium chloride that is making the claim on its product’s label that it heals cold sores. The FDA found that the active ingredient, benzalkonium chloride, is not indicated as a cold sore treatment and may not make cold sore healing claims because there is no scientific evidence to support claims that it heals cold sores.
Evidence suggests that Abreva shortens the duration of cold sores by about 18 hours, and may slightly reduce the risk of outbreak if the medicine is used at the first sign of pain or tingling. Whether that’s worth the price of the treatment is up to the consumer, but choosing a cheaper product with a different active ingredient is likely to be a waste of money.
The Abreva case serves as a reminder to check the active ingredients in your OTC medicines before purchasing what seems to be a cheaper, equivalent medicine. While generics are often a smart way to save money on effective medicines, look-alike medicines can sometimes be hiding fake cures in a convincing little package. Let the buyer beware!
April 25th, 2011 by David Kroll, Ph.D. in News
No Comments »

After spending the first 21 years of life in New Jersey and Philadelphia, I ventured to the University of Florida for graduate school. For those who don’t know, UF is in the north-central Florida city of Gainesville – culturally much more like idyllic south Georgia than flashy south Florida.
It was in Gainesville – “Hogtown” to some – that I first encountered the analgesic powder. I believe it was BC Powder, first manufactured just over 100 years ago within a stone’s throw of the Durham, NC, baseball park made famous by the movie, Bull Durham. I remember sitting with my grad school buddy from Kansas City watching this TV commercial with hardy men possessing strong Southern accents enthusiastically espousing the benefits of BC. I looked at Roger – a registered pharmacist – and asked, “what in the hell is an analgesic powder?”
What I learned is that powders of analgesic compounds were one of the individual trademark products of Southern pharmacies during the early 1900s. Many of these powders became quite popular with mill and textile workers needing to calm headaches induced by long hot days with loud machinery. The original powders contained a precursor to acetaminophen called phenacetin. However, phenacetin was found to cause renal papillary necrosis, such as in this 1964 case report in Annals of Internal Medicine.
Today, most of these powders are comprised of aspirin, acetaminophen, and caffeine. Read more »
*This blog post was originally published at Science-Based Medicine*
July 12th, 2010 by AndrewSchorr in Better Health Network, Health Policy, News, Opinion, Research
No Comments »

They have a tough job, those government doctors, scientists, and bureaucrats who are charged with assessing the safety and effectiveness of proposed new medical products. As you know, they rely largely on studies presented by the applicants.
The Food and Drug Administration (FDA) has the power to not approve a new drug or product or even pull it off the market. Right now it is considering limiting or pulling GlaxoSmithKline’s (GSK) diabetes drug, Avandia, because of newly discovered data that it may have caused heart attack in some patients –- data mysteriously not shown in GSK’s own studies. If the drug is pulled it will cost GSK billions of dollars in lost revenue but, from the FDA’s point-of-view, it will be protecting the public. And, after all, there are safer diabetes drugs on the market as alternatives. Read more »
*This blog post was originally published at Andrew's Blog*
February 9th, 2010 by PhilBaumannRN in Better Health Network, Opinion
No Comments »


- Image via Wikipedia
Was your company blogging ten years ago? If not then why? Google made it easy for you and now you’ve lost ten years of priceless link juice. Given the fragmentation of media in the last ten years, it’s clear now just how relatively little work you actually had to do back then. But that’s in the past. Still, I have bad news for you: what you have to do now is far harder than it was ten years ago. Let me explain.
CONTENT FRAGMENTATION AND SOCIAL DISTORTION
As the Web expands and proliferates novel media, messaging becomes increasingly diffuse and fragmented. The Web creates new opportunities and destroys old standards. It disrupts communication patterns, rattles social structures and ruptures attention spans. Ten years ago, you could leverage your audience-building skills for acquiring and retaining customers. You could even have learned and mastered a skill which traditional marketing didn’t really demand: conversational aptitude. Read more »
*This blog post was originally published at Phil Baumann*
 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).













