December 18th, 2011 by Toni Brayer, M.D. in Health Tips, News
2 Comments »

 Women have been told they should have screening for cervical cancer with a pap test every year. The visit to the gynecologist or internal medicine physician has been a right of passage for most young women and most are very compliant with that annual visit throughout their lives.
Women have been told they should have screening for cervical cancer with a pap test every year. The visit to the gynecologist or internal medicine physician has been a right of passage for most young women and most are very compliant with that annual visit throughout their lives.
Well, the times they are a-changin’ because new guidelines issued by the US Preventative Services Task Force and the American Cancer Society say women should undergo screening NO MORE OFTEN than every 3 years starting at age 21. To further strengthen this recommendation, even the American Society for Clinical Pathology (those folks that read the pap smears) agrees with the recommendation. They also recommend stopping routine pap smears after age 65 for women who have had 3 negative Pap test results in the past 10 years. These women are just not at high risk.
So why the change? Read more »
*This blog post was originally published at EverythingHealth*
December 3rd, 2011 by Elaine Schattner, M.D. in Opinion, Research
No Comments »

The latest issue of the Annals of Internal Medicine contains 2 noteworthy papers on cervical cancer screening. The first, a systematic review of studies commissioned by the USPSTF, looked at 3 methods for evaluating abnormalities in women over 30 years:
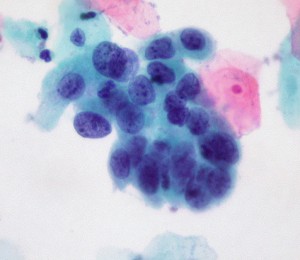
high-grade cervical cell dysplasia (Dr. E. Uthman, Wikimedia Commons)
1. Conventional cytology (as in a Pap smear; the cervix is scraped and cells splayed onto a microscope slide for examination);
2. Liquid-based cytology (for LBC, the NHS explains: the sample is taken as for a Pap test, but the tip of the collection spatula is inserted into fluid rather than applied to slides. The fluid is sent to the path lab for analysis);
3. Testing for high-risk HPV (human papillomavirus). Currently 3 tests have been approved by the FDA in women with atypical cervical cells or for cervical cancer risk assessment in women over the age of 30: Digene Hybrid Capture 2 (manufactured by Quiagen), Cobas 4800 HPV (Roche) and Cervista HR HPV (Hologic); another Roche Diagnostics assay, Amplicor HPV, awaits approval.
These HPV assays use distinct methods to assess DNA of various HPV strains.
There’s a lot of jargon here, and I have to admit some of this was new to me despite my nearly-due diligence as a patient at the gynecologist’s office and my familiarity as an oncologist with the staging, clinical manifestations and treatment of cervical cancer. Who knew so many decisions were made during a routine pelvic exam about which manner of screening? Read more »
*This blog post was originally published at Medical Lessons*
November 17th, 2011 by Lucy Hornstein, M.D. in Opinion
No Comments »

Cancer. Just the word is scary. Actually, that’s the problem. Once you say that word, the average American will do anything — ANYTHING! — to just get it out of my body!!! Whether or not they have it, whatever the actual numerical chances of their ever developing it, no chance for detecting or treating it should ever be neglected. EVER! Ask any Med-mal lawyer. Better, ask any twelve average people off the street (i.e., the ones who are going to wind up on a jury). “The doctor didn’t do every possible test/procedure, and now the patient has CANCER? String him up!”
Hence we have the new guidelines for PSA testing. (Given that many patients with prostate cancer have normal PSAs and lots of patients with high PSAs don’t have prostate cancer, it doesn’t seem semantically correct to call it “prostate cancer screening”.) Surprise! Turns out that not only does PSA testing not save lives, but that urologists don’t really care. Certainly not enough to stop recommending PSAs to just about everyone they can get their hands on.
Nor do breast surgeons have any intention of modifying their recommendations, not only in light of new understandings of the limitations of mammography, but even as Read more »
*This blog post was originally published at Musings of a Dinosaur*
November 2nd, 2011 by Peggy Polaneczky, M.D. in Health Policy, Opinion
No Comments »

 On November 8, Mississippians will be voting on ballot amendment 26 , the so called “Personhood Amendment” that if passed, would declare a fertilized egg a person.
On November 8, Mississippians will be voting on ballot amendment 26 , the so called “Personhood Amendment” that if passed, would declare a fertilized egg a person.
The question at hand is, would the Personhood Amendment be used to outlaw contraception?
Dr. Freda Bush, an Ob-Gyn and spokesperson for the Personhood amendment in Mississippi, seems to think it will not. In a press conference in support of the amendment in September, she stated this –
The personhood amendment will not ban the use of hormonal contraceptives.
The video of this press conference is being used to reassure voters about the intent of amendment 26. And yet the information Dr. Bush presents about contraception and the amendment stands in complete contrast to that which the personhood movement itself has presented. Here is the standard “talking point” on contraception from personhood sites at states across the country seeking to pass similar amendments – Read more »
*This blog post was originally published at The Blog That Ate Manhattan*
September 21st, 2011 by Peggy Polaneczky, M.D. in Opinion
No Comments »

 Just yesterday, I put up a post about the recent birth control pill recall. This recall is a big deal – millions of women are potentially impacted, and the adverse effect – an unplanned pregnancy – is very significant.
Just yesterday, I put up a post about the recent birth control pill recall. This recall is a big deal – millions of women are potentially impacted, and the adverse effect – an unplanned pregnancy – is very significant.
I knew women taking these pills would be very worried, and wanted very much to do more than just spit out the press release from the FDA. I wanted to both reassure women and give them information that they could use other than just a link and a phone number. I also needed to figure out how I would be handing the recall in my own practice. So I combined the two and posted what I’ll be telling my patients to do if they find that they are taking a recalled pill pack.
As soon as the post went up, I got worried.
What if the advice I was giving my patients was not what other docs might do for their patients? Read more »
*This blog post was originally published at The Blog That Ate Manhattan*
 Women have been told they should have screening for cervical cancer with a pap test every year. The visit to the gynecologist or internal medicine physician has been a right of passage for most young women and most are very compliant with that annual visit throughout their lives.
Women have been told they should have screening for cervical cancer with a pap test every year. The visit to the gynecologist or internal medicine physician has been a right of passage for most young women and most are very compliant with that annual visit throughout their lives.
















