October 20th, 2015 by Dr. Val Jones in Health Tips, Opinion
1 Comment »
 Most of my patients think about pain medicines in terms of the symptoms they treat. “This is my headache medicine, and this is my arthritis medicine,” they often say. Healthcare providers are more likely to categorize pain medicines by the way they work: some are anti-inflammatory, some affect nerve endings, and others influence how the brain perceives pain. But the truth is that no matter how you classify pain medicines, there is no way to know if they’ll help until you try them for yourself.
Most of my patients think about pain medicines in terms of the symptoms they treat. “This is my headache medicine, and this is my arthritis medicine,” they often say. Healthcare providers are more likely to categorize pain medicines by the way they work: some are anti-inflammatory, some affect nerve endings, and others influence how the brain perceives pain. But the truth is that no matter how you classify pain medicines, there is no way to know if they’ll help until you try them for yourself.
Most people don’t realize that pain management is personal. Research is beginning to help us understand why people respond to medicines so differently, and one day we will probably be able to personalize treatment plans more successfully. For now, there are several known genetic reasons why pain medicines are more or less effective for one individual over another. Genes affect:
-
The number of enzymes that break down medicines and remove them from the body. Some people have larger numbers of these enzymes and therefore require more drug to feel its pain-relieving effects. Others may be strongly affected by even small doses of drug.
-
Pain medicine receptor variations can make one medicine effective and another (nearly identical medicine) ineffective in relieving pain.
-
Differences in carrier molecules that transport pain medicine across the blood stream and into the cells that are triggering pain sensations. Some people have fewer carrier molecules to bring the medicine to the site of pain.
-
The number of “middle man” neurotransmitter molecules that pass along the pain response. Too many of these molecules can reduce drug binding and mute the pain relief effects of some drugs.
When pain is severe, prescription medications may be necessary. However, mild to moderate pain may be effectively managed with over-the-counter (OTC) medicines. I believe in the start low, go slow approach to finding the smallest effective dose of pain medicines. I always recommend that my patients read and follow all the instructions on the Drug Facts labels to make sure that they don’t accidentally overdose on active ingredients.
When I choose a pain reliever with my patients, the first thing I think about is potential side effects. Some medicines (such as non-steroidal anti-inflammatory drugs like ibuprofen and naproxen sodium) can be hard on the stomach lining, or cause bleeding in people who are at risk for it. Other medicines (such as acetaminophen) can harm the liver if used in excess, while prescription pain medicines can cause constipation and drowsiness. The best pain medicine to start with is one that is least likely to cause harm to the specific person.
The next thing I ask is whether or not the medicine has worked for the patient in the past. Previous experience is one of the best indicators of future success. Since I know that my patient has a unique, genetically determined number of enzymes, transporters, and receptors, previous experience with pain medicines will give me a good idea of how well they will tolerate it again, and if it will be effective.
Finally, I consider the type of pain that they are experiencing. If the pain is caused by inflammation (from an injury, surgery, or arthritis) I’ll consider a medicine with primarily anti-inflammatory properties. If the pain is caused by tension (such has headache) or complicated by fever, I may consider acetaminophen first. If the pain is coming from a nerve (such as sciatica or neuropathy) then I’ll use pain medicines that work for nerve pain specifically. If the pain is complicated by depression, I may discuss additional medicines and approaches.
Sometimes, combinations of medicines are significantly more effective than one medicine alone at treating pain (this is why some prescription pain relievers are combinations of an opioid and acetaminophen). When using more than one pain relief medicine, it is important to compare active ingredients in both prescription medications and OTC products to make sure that accidental overdoses do not occur. I also recommend consulting with a healthcare professional if there are concerns about drug interactions or if the patient is already on a significant number of prescription medications that could interact with his or her OTC pain medicine choices.
The bottom line is that science is still catching up to pain management. Perhaps one day a simple blood test will help us to determine the very best pain medicine regimen for a specific patient at a given time. But until then, adopting a strategy of careful trial and error (avoiding unwanted side effects, using the lowest effective doses, and consulting a physician when pain is severe) is the only option. Don’t worry too much about whether a specific medicine is “best” for your pain. Pain management is very personal, so you will need to discover your own best solution.
***
Disclosure: Dr. Val Jones is a paid consultant for McNeil Consumer Healthcare Division.
July 10th, 2012 by Dr. Val Jones in Health Tips, News
No Comments »
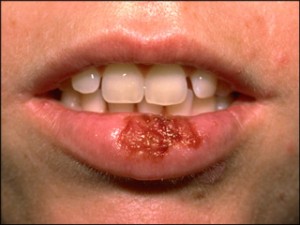 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to shorten the duration as well as prevent the outbreak of cold sores (an estimated 20-40% of Americans are afflicted by cold sores).
In a recent survey (sponsored by Abreva), 66% of respondents said they believed that “look-alike” medications contained the same active ingredients as the brands that they were copying, and 93% said they purchased look-alikes solely because they were less expensive.
Unfortunately, you get what you pay for in this case. Even though the FDA may send out warning letters to manufacturers of look-alike products who print false healing claims on their packaging, those products often remain on store shelves. The manufacturer of Abreva (Glaxo Smith Kline) notes in a press release:
Several “look-alike” cold sore treatments tout healing claims, but contain the ingredient Benzalkonium Chloride instead of docosonal. The FDA recently issued a warning letter to a marketer and distributor of a product containing benzalkonium chloride that is making the claim on its product’s label that it heals cold sores. The FDA found that the active ingredient, benzalkonium chloride, is not indicated as a cold sore treatment and may not make cold sore healing claims because there is no scientific evidence to support claims that it heals cold sores.
Evidence suggests that Abreva shortens the duration of cold sores by about 18 hours, and may slightly reduce the risk of outbreak if the medicine is used at the first sign of pain or tingling. Whether that’s worth the price of the treatment is up to the consumer, but choosing a cheaper product with a different active ingredient is likely to be a waste of money.
The Abreva case serves as a reminder to check the active ingredients in your OTC medicines before purchasing what seems to be a cheaper, equivalent medicine. While generics are often a smart way to save money on effective medicines, look-alike medicines can sometimes be hiding fake cures in a convincing little package. Let the buyer beware!
January 5th, 2012 by Jeffrey Benabio, M.D. in Health Tips
No Comments »


If your legs are red, bumpy, and irritated, don’t blame your soap or body lotion. It may be your skinny jeans. Those popular body-huggers may look hot, but they could be causing some very uncool things on your skin.
When you move in your skinny jeans, the material causes friction on your skin, which over time, can lead to folliculitis, inflammation of the hair follicles. If you have red, itchy bumps or pimples, especially on your thighs where skinny jeans tend to be tightest, then stop wearing them for a few days to let your skin heal. To treat folliculitis, Read more »
*This blog post was originally published at The Dermatology Blog*
December 14th, 2011 by AmyGivlerMD in Health Tips
3 Comments »


Many of my patients, over the years, have taken melatonin. Many other patients have asked me about it, but I’ve never had much to say. I hadn’t heard anything particularly bad about it, but couldn’t really recommend it. “Research melatonin” has been on my “To Do” list for a long time.
So here’s what I’ve discovered: Melatonin is a hormone. I’ve known that since medical school, of course, but that fact has struck me as peculiar these past few weeks. Why? Because it’s sold over the counter, and many people take massive amounts of it. No other hormone is available like this. The use of other hormones, such as insulin and thyroid hormone, need careful monitoring. Is melatonin so universally safe that it can be taken at any dose, for however long? The more we learn about melatonin, the less that seems to be the case. Read more »
*This blog post was originally published at Making Sense of Medicine*
December 9th, 2011 by BarbaraFederOstrov in Health Policy, News
No Comments »
There’s plenty of of analysis, criticism and praise of HHS Secretary Kathleen Sebelius’ controversial decision to prevent the “morning after” contraceptive pill Plan B from being sold over the counter at drugstores and to girls under 17 without a prescription. The top question: how much did election-year politics affect the decision?
President Barack Obama, father of two daughters, defended Sebelius today and said he was not involved in her decision. The New York Times quotes him:
The reason Kathleen made this decision is that she could not be confident that a 10-year-old or an 11-year-old going to a drug store should be able — alongside bubble gum or batteries — be able to buy a medication that potentially, if not used properly, could have an adverse effect.
Here’s a roundup of the national conversation so far:
NPR’s Julie Rovner reports today on the angry reactions from women’s health advocates, who note that Sebelius’ reasoning – that young girls might not use the OTC birth control correctly – sets a double standard for birth control. She quotes former assistant FDA commissioner Susan Wood: Read more »
*This blog post was originally published at Reporting on Health - Barbara Feder Ostrov's Health Journalism Blog*
 Most of my patients think about pain medicines in terms of the symptoms they treat. “This is my headache medicine, and this is my arthritis medicine,” they often say. Healthcare providers are more likely to categorize pain medicines by the way they work: some are anti-inflammatory, some affect nerve endings, and others influence how the brain perceives pain. But the truth is that no matter how you classify pain medicines, there is no way to know if they’ll help until you try them for yourself.
Most of my patients think about pain medicines in terms of the symptoms they treat. “This is my headache medicine, and this is my arthritis medicine,” they often say. Healthcare providers are more likely to categorize pain medicines by the way they work: some are anti-inflammatory, some affect nerve endings, and others influence how the brain perceives pain. But the truth is that no matter how you classify pain medicines, there is no way to know if they’ll help until you try them for yourself.

 Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to
Many people assume that look-alike over-the-counter (OTC) medicines contain the same active ingredients as the more expensive brand name products. But that’s not always the case. Take lip medicines for example – many of their labels suggest that they treat cold sores (caused by the herpes simplex virus), but only one active ingredient has been proven to work. Docosanol is the active ingredient in Abreva, and has been approved by the FDA to 











