December 19th, 2008 by Dr. Val Jones in Opinion
No Comments »
I was reading a good blog post about ethics violations in clinical trials in India, and was reminded of an interesting case in the U.S. Several years ago a 21-year-old woman (Abigail Burroughs) with terminal cancer (who had exhausted all treatment options) was denied the option of purchasing a drug that was in the final process of receiving FDA approval. The FDA ruled that terminally ill patients should not be permitted to purchase drugs until the drugs have completed the approval process, citing safety concerns. Their arguments also included that:
1. Patients cannot make a truly informed decision regarding whether or not to try a medicine that has not yet been fully tested.
2. Allowing access to drugs before they are FDA approved would undermine the clinical trial process.
3. If terminally ill patients could buy treatments, others would soon expect health plans to cover these costs for others.
4. Allowing access to drugs before they were proven effective would add to the public’s general confusion about evidence-based medicine and promote magical thinking.
I don’t know how you feel about this, but it makes me squirm a little bit. Although I appreciate the FDA’s position (and my colleague Dr. Jim Sabin has blogged about his support for the ruling) it just seems a little heavy-handed, especially in light of the details of the Abigail case. Here’s what I wrote to Dr. Sabin:
About the Abigail case… I think they were trying to get access to Erbitux for Abigail, and it was in phase III trials at the time (so there was mounting evidence for its efficacy – their request to purchase it wasn’t scientifically unreasonable.)
I have very mixed feelings about the FDA ruling. I understand that we don’t want to 1) set a precedent that would lead to insurers having to pay for expensive experimental therapies 2) discourage people from entering clinical trials 3) allow drug makers to profit from “false hopes” in terminal cases. However, couldn’t we add enough caveats to make it reasonable to allow patients who have exhausted all other options to purchase (with their own money) drugs that have shown promise but are not yet FDA approved?
What if we said that the drugs had to be in phase III trials, with enough evidence to suggest a plausible benefit for the patient? Terminal patients are rarely good candidates for clinical trials (I don’t think we’d be poaching from the CT pool), few can afford to buy monoclonal antibodies (and the like) on their own so if there were trials for terminal patients, they’d still have great incentive to join, and I’m not sure that just because a patient can buy a treatment that insurance will HAVE TO follow suit.
Something about limiting personal choices (for those who have the means to make them) seems un-American to me. There are very low risks of harm in monoclonal antibody treatments (so the argument that the FDA must err on the side of patient safety doesn’t really fit the Abigail case). Yes, it’s sad for those who can’t afford to buy every possible therapy – but why should we deny access for those who can? With the right caveats, I think we could allow people like Abigail to try every remaining option. But of course I agree that phase I drugs are just not far enough along the research pipeline to make educated choices about them.
Maybe Abigail was a casualty of the one-size-fits-all, population-based rules that are appropriate most of the time, but fail in exceptional cases. I predict that this sort of thinking (where evidence-based protocols are applied in a cook book manner to all patients with a given disease) will dominate the healthcare landscape in the coming years as we seek to reign in costs and do the best thing for most. I just wish there were a way to bend rules on the edges a bit. What do you think?
November 17th, 2008 by Dr. Val Jones in Announcements
4 Comments »
I was a panelist at Edelman’s CHPA New Media Summit today in New Brunswick, NJ. Matthew Holt (of the Healthcare Blog and Health 2.0) was the keynote speaker, and I participated on a panel discussion with Dr. Roy Poses. It was exciting to meet Roy in person for the first time, as I’ve been following his policy blog for some time.
Matthew presented a very rosy picture of Health 2.0 (consumer-driven healthcare), more or less suggesting that it could provide a large part of the solution to our current healthcare crisis. I countered with a more cautious view, explaining that expert engagement would be critical to Health 2.0’s success.
Matthew argued that sites like Patients Like Me were enabling patients “to do their own clinical trials online,” and that this was opening a whole new avenue for research. Dr. Poses and I were fairly concerned about this suggestion, primarily because we understand how easy it is to draw false conclusions from data, especially when the data are not collected in a systematic fashion.
I explained to the audience that association does not prove causation (E.g. Do matches cause lung cancer? No, though it’s true that people who smoke are more likely to carry matches). I also described a case in which a Health 2.0 principle went terribly awry: a group of consumers were asked to rate their medications for efficacy by disease/condition. This was supposed to leverage the “wisdom of the crowds” to determine which medicines worked best (by popular vote). Of course, the result was that every pain syndrome (low back pain, headaches, fibromyalgia, etc.) resulted in a narcotic pain medicine as the highest ranked treatment option. Do you really need Oxycontin for that tension headache of yours? Obviously, narcotics are popular among users – but are a last resort for pain management in the real world. The “wisdom of crowds” rarely works in healthcare.
Matthew agreed to disagree with me on a number of issues – but we certainly found common ground on the primary care crisis. He and I both believe that a shortage of primary care physicians is going to result in a catastrophic shortage of care for Baby Boomers in the next decade. Dr. Poses added that primary care physicians make the same salary as school teachers in his home state of Rhode Island.
I think we have to agree with KevinMD – the way forward is not going to be pretty.
November 4th, 2008 by Dr. Val Jones in Opinion, True Stories
5 Comments »
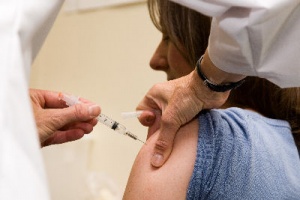
Election anxiety has America on the edge of its seat. I anticipated long lines and a lot of drama, so I voted early to avoid the rush. That left me with nothing election-related to do today, so I decided to head over to my local pharmacy and get a flu shot instead.
Last year the flu vaccine was only 50% effective because experts did not correctly predict which viral strains would victimize Americans. This year I have my fingers crossed that the Brisbane and Florida strains included in the vaccine will do the trick. After all, Influenza is the single leading cause of vaccine-preventable disease in the U.S., with estimates between 15 million and 60 million cases in the US a year among all age groups. Influenza leads to 200,000 hospitalizations and about 36,000 deaths a year in the U.S., mostly in infants and the elderly. I’ll never forget the touching story of how one family lost their three and-a-half year-old daughter to the flu.
So I arrived at the pharmacy only to find a disorderly group of flu-shot seekers, pacing near the entrance to the retail clinic. About 20 minutes later a young woman with a clipboard and sign up sheets came out and started asking people what kind of insurance they had. When my turn came she informed me that my insurance plan was not participating, and suggested that I leave. I asked if I could pay out-of-pocket for the shot and she said that I could and gave me a consent form. More people arrived without any movement in the line, and I overheard one person commenting that the nearby polling booth wasn’t moving as slowly. Another customer decided to leave to go vote and then come back later for the shot.
Forty minutes later my name was called and I entered a small room littered with papers and syringe caps. I rolled up my right sleeve and asked the technician about his injection technique. I watched him carefully draw up half a cc of vaccine from a multiple-use bottle.
He then asked me how I was going to pay. I presented my credit card and he said that he only accepted cash or check. I said that I had no idea that credit cards weren’t accepted and he seemed surprised that I wasn’t aware of the retail clinic policy. A large envelope was leaning against his chair leg, full of $30 cash deposits for the shot. Read more »
October 28th, 2008 by Dr. Val Jones in Expert Interviews, Health Policy
No Comments »
 |
|
Bert Rein
|
On November 3, 2008 the US Supreme Court will hear opening arguments in the Wyeth vs. Levine case. This highly publicized lawsuit has been discussed by the New York Times and the Journal of the American Medical Association and will likely be the most important case during the upcoming Supreme Court term. However, neither source has fully explained the unexpected consequences to the consumer if Wyeth loses.
To get to the bottom of the issue, I interviewed Bert Rein, attorney for Wyeth. Bert has conducted interviews with NPR and the three major TV news networks. Please enjoy this exclusive podcast interview here at Getting Better with Dr. Val, or read my summary of our conversation below.
Dr.Val: Bert, please summarize for our listeners what has happened so far in the Wyeth vs. Levine case.
Rein: Ms. Levine is a guitarist who suffers from migraine headaches and associated nausea. One day she sought pain management therapy at a clinic in northeast Vermont – the same clinic where she regularly received care. They elected to treat her with a combination of demerol (for pain) and phenergan (for nausea). They delivered the drugs intramuscularly, but several hours later Ms. Levine returned, complaining of an unrelieved migraine headache.
The clinic’s physician realized that the drugs would be more potent if they were injected intra-venously so he asked the PA (physician assistant) to give another dose of the drugs through Ms. Levine’s vein. Unfortunately, the PA inserted a butterfly needle (rather than the usual heplock for an IV) into what she thought was Ms. Levine’s vein, and delivered the phenergan into or near a punctured artery. Phenergan’s label clearly states that the drug can cause tissue necrosis if it comes in contact with arterial blood. Ms. Levine experienced a necrotic reaction to the medication which resulted in the eventual amputation of her arm. She sued the clinic for negligence and was awarded $700,000 dollars in a cash settlement.
Ms. Levine then brought a separate lawsuit against Wyeth, claiming that the phenergan label did not offer sufficient instructions about how to administer it safely, though the risks of necrosis from arterial blood exposure to phenergan are well known and labeled in capital letters as a warning on the drug’s label. Read more »
October 7th, 2008 by Dr. Val Jones in Medblogger Shout Outs
No Comments »
Thanks to Dr. Wes for hosting me during my recent period of blog homelessness. Please check out his excellent site – here’s my featured post:
It seems in cardiology, things are so tiny: tiny angioplasty balloons, itsy bitsy guidewires to snake down the smallest of coronary arteries. Heck most things they deal with are measured in millimeters: need I say more? Now electrophysiologists, well, I’ve already had my say.
But Dr. Val, today’s guest blogger who transitions from her old space at Dr. Val and The Voice of Reason at Revolution Health to her new site at Getting Better With Dr. Val, notes a new trend in medical marketing to these marvelous medics of the miniature: