June 13th, 2009 by RamonaBatesMD in Better Health Network, Health Tips
2 Comments »

Last Tuesday, this tweet from @AllergyNotes caught my eye.
 Call cubital tunnel syndrome a “cell phone elbow” and you make the front page of CNN.com: http://bit.ly/RaXrt and http://bit.ly/TTRfg
Call cubital tunnel syndrome a “cell phone elbow” and you make the front page of CNN.com: http://bit.ly/RaXrt and http://bit.ly/TTRfg
Cubital tunnel syndrome I know, but I had not heard it called “cell phone elbow.” The first link is to the Cleveland Clinic Journal of Medicine article (full reference below). It is an excellent article and well worth reading. The second link is to CNN news article picking up the “cell phone elbow” line.
Cubital tunnel syndrome is a nerve compression syndrome (like carpal tunnel syndrome). In the case of cubital tunnel syndrome, the nerve involved is the ulnar nerve and the location is at the elbow. From the article
… the ulnar nerve as it traverses the posterior elbow, wrapping around the medial condyle of the humerus. When people hold their elbow flexed for a prolonged period, such as when speaking on the phone or sleeping at night, the ulnar nerve is placed in tension; the nerve itself can elongate 4.5 to 8 mm with elbow flexion……..
As with other nerve compression syndromes, the clinical picture is representative of the nerves enervation. In the case of the ulnar nerve, this involves numbness or paresthesias in the small and ring fingers. There may also be numbness of the dorsal ulnar hand which will NOT be present if the ulnar nerve compression is in the Guyon’s canal at the wrist level (distal ulnar nerve compression). If the compression is chronic enough, the symptoms progress to hand fatigue and weakness. The small intrinsic muscles of the hand are important in hand strength needed to open jars. More from the article
Chronic and severe compression may lead to permanent motor deficits, including an inability to adduct the small finger (Wartenberg sign) and severe clawing of the ring and small fingers (a hand posture of metacarpophalangeal extension and flexion of the proximal and distal interphalangeal joints due to dysfunction of the ulnar-innervated intrinsic hand musculature). Patients may be unable to grasp things in a key-pinch grip, using a fingertip grip instead (Froment sign).
It may be an old joke (Patient: Doctor, it hurts when I do this. … Doctor: Well don’t do it.), but in the case of cubital tunnel syndrome it fits. Prevention is key. Prolonged extreme flexion of the elbow (elbows bent tighter than 90 degrees) is not kind to the ulnar nerve. Switch hands or use a head set or blue tooth.
REFERENCES
Q:What is cell phone elbow, and what should we tell our patients?; Cleveland Clinic Journal of Medicine May 2009 vol. 76 5 306-308 (doi: 10.3949/ccjm.76a.08090); Darowish, Michael MD, Lawton, Jeffrey N. MD, and Evans, Peter J MD, PhD
Cubital Tunnel Syndrome: eMedicine Article, Feb 9, 2007; James R Verheyden, MD and Andrew K Palmer, MD
*This blog post was originally published at Suture for a Living*
May 21st, 2009 by Dr. Val Jones in Health Tips, True Stories
No Comments »
There’s no technological substitute for the human hand. Manual dexterity is incredibly hard to replicate, and so surgeons will go to great lengths to save injured hands. Unfortunately, sometimes the injury is too severe to allow for any meaningful functional recovery.
In these two cases, well-meaning surgeons refused to amputate the unsalvageable hands, thus delaying recovery and adaptation of prostheses.
This is a photo of a trauma victim who underwent extensive reconstruction of the hand, including transplantation of a toe to the thumb’s position. Gangrene set in and tracked up one of the tendon sheaths.
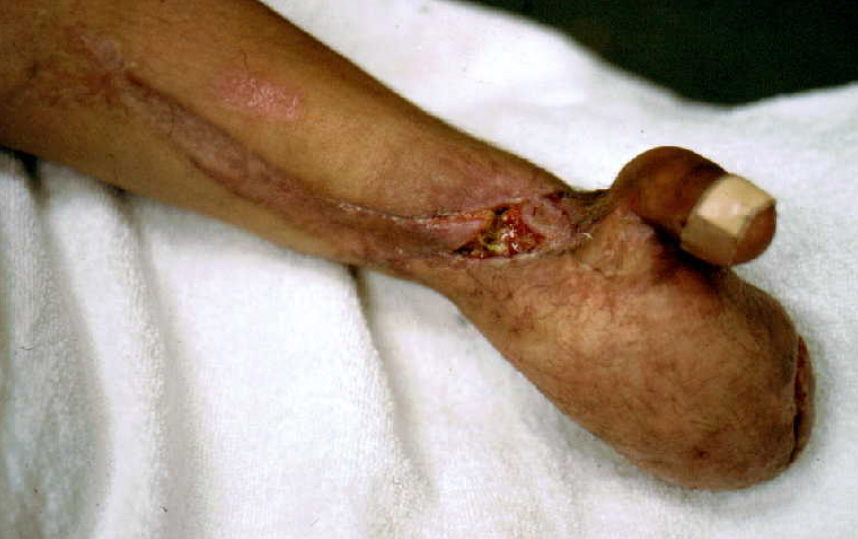
Photo Credit: Dr. Heikki Uustal
In this case, a burn victim was hoping to have some fingers reconstructed from his fist. He declined amputation and fitting with a prosthesis, despite the potential for enhanced function.
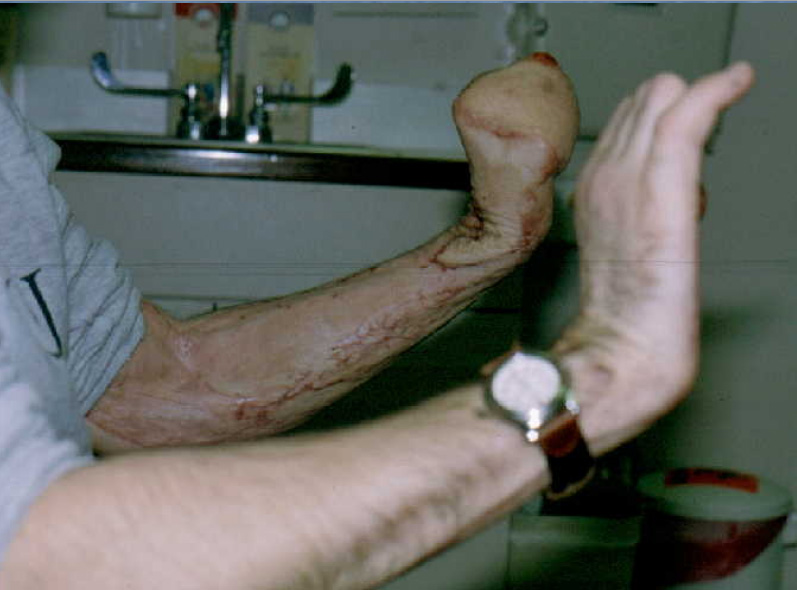
Photo Credit: Dr. Heikki Uustal
In both cases, a wrist disarticulation (amputation at the wrist) and prosthetic fitting (such as this myo-electric device with a self-suspending socket) might have provided a better functional and cosmetic outcome:
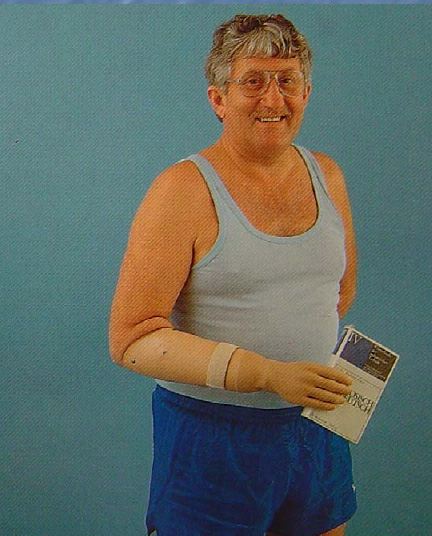
Photo Credit: Dr. Heikki Uustal
Sometimes, it’s better to amputate.
May 12th, 2009 by RamonaBatesMD in Better Health Network
No Comments »

The U.S. Food and Drug Administration recently announced that safety label changes including a boxed warning and a Risk Evaluation and Mitigation Strategy (REMS) will now be required for all botulinum toxin products. The agency took the action because of two main reasons. The first one is the potential for serious risks that may occur from the spread of the botulinum toxin beyond the injection site. The second reason is associated with the lack of interchangeability among the three licensed botulinum toxin products.
When the botulinum toxin spreads beyond the area of injection, symptoms similar to botulism may occur. These symptoms include unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids.
This “spreading effect” has been reported in both children and adults. It has been reported most often in children with cerebral palsy being treated with the products for muscle spasticity. Treatment of muscle spasticity is an off-label use of the drug. The “spreading effect” has been reported in patients being treated for both approved and unapproved uses.
Botulinum toxin products include:
-
Botox and Botox Cosmetic (botulinum toxin type A), marketed by Allergan
-
Myobloc (botulinum toxin type B), marketed by Solstice Neurosciences
-
Dysport (abobotulinumtoxinA), a new FDA-approved product marketed by Ipsen Biopharm Ltd.
All are approved by the FDA for the treatment of cervical dystonia. Botox Cosmetic and Dysport are approved by the FDA for treatment of glabellar frown lines. Botox is approved for the treatment of severe underarm sweating (primary axillary hyperhidrosis), crossed eyes (strabismus), and abnormal tics and twitches of the eyelids (blepharospasm). All other uses are considered off-label.
The FDA has not identified any definitive serious adverse event reports of a distant spread of toxin effect producing symptoms consistent with botulism when these products are used in accordance with the approved label.
It is important for those of us who use botulinum toxins to remember that the dosage strength between the products is not the same. One unit of Botox is not equal in strength (potency) as one unit of Myobloc or Dysport.
BOTOX® Cosmetic Insert (pdf)
Dilution Technique: Using a 21-gauge needle and an appropriately sized syringe draw up a total of 2.5 mL/100 Unit vial or 1.25 mL/50 Unit vial of 0.9% sterile saline without a preservative…….
Injection Technique: Glabellar
Using a 30-gauge needle, inject a dose of 0.1 mL into each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 20 Units. Typically the initial doses of reconstituted BOTOX® Cosmetic induce chemical denervation of the injected muscles one to two days after injection, increasing in intensity during the first week.
MYOBLOC® Insert (pdf)
The recommended initial dose of MYOBLOC® for patients with a prior history of tolerating botulinum toxin injections is 2500 to 5000 U divided among affected muscles. Patients without a prior history of tolerating botulinum toxin injections should receive a lower initial dose. The duration of effect in patients responding to MYOBLOC® treatment has been observed in studies to be between 12 and 16 weeks at doses of 5000 U or 10,000 U.
Dysport® Insert
The units of Dysport are specific to the preparation and are not interchangeable with other preparations of botulinum toxin.
Glabellar lines Dosage
The dosage is dependant on the severity of the lines and the specific muscle being treated.
For the corrugator and procerus muscles 40 to 60 units divided between injection sites as follows:
8 to 12 units in each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 60 units
Improvement of severity of glabellar lines generally occurs within 72 hours after treatment and persists for 3 to 6 months.
It is important for us to educate patients and their caregivers of the potential adverse effects. Some of these effects have been reported as early as several hours and as late as several weeks after treatment. Patients should seek immediate medical attention if they develop any of these symptoms — unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids.
Adverse events may be reported by health care professionals and/or consumers to the FDA’s MedWatch Adverse Event Reporting program by four ways:
REFERENCES
FDA News (April 30, 2009)
BOTOX® Injections; eMedicine Article, Sept 25, 2008; Robert A Hauser, MD, MBA, Mervat Wahba, MD, Theresa McClain, MSN, ARNP
*This blog post was originally published at Suture for a Living*
April 28th, 2009 by Dr. Val Jones in True Stories
No Comments »
Doctor: Mr. Smith, your urine test is positive for cocaine.
Mr. Smith: [Blank Stare]
Doctor: Have you been snorting cocaine recently?
Mr. Smith: No.
Doctor: Then why is there cocaine in your urine?
Mr. Smith: Maybe your nurse put it in there.
Doctor: If my nurse had cocaine, I don’t think she’d put it in your urine.
***
Bonus tip for pain management specialists: cocaine’s half-life in the urine is 2-4 days. “Random” urine drug testing on Mondays offers a higher yield than other days of the business week because most patients abuse illicit drugs on weekends>>weekdays.
April 22nd, 2009 by Medgadget in Better Health Network
1 Comment »
 A five year old British girl who had her outer limbs amputated due to meningitis (meningococcemia with meningitis accompanied by gangrene of the extremities would be our guess) has received a new pair of legs.
A five year old British girl who had her outer limbs amputated due to meningitis (meningococcemia with meningitis accompanied by gangrene of the extremities would be our guess) has received a new pair of legs.
The high tech carbon fiber pair is of the variety commonly seen on competitive Special Olympics athletes, some of whom run faster than old fashion legged people. Ellie’s parents say that she already walks twice as fast as her previous conventional prosthetic pair.
We believe that medical devices will greatly improve Ellie’s life in the future, and hopefully she can one day receive a proper pair of Deka arms.
More from Echo UK…
(hat tip: Gizmodo)
*This post was originally published at Medgadget.com*
 Call cubital tunnel syndrome a “cell phone elbow” and you make the front page of CNN.com: http://bit.ly/RaXrt and http://bit.ly/TTRfg
Call cubital tunnel syndrome a “cell phone elbow” and you make the front page of CNN.com: http://bit.ly/RaXrt and http://bit.ly/TTRfg





 A five year old British girl who had her outer limbs amputated due to meningitis (meningococcemia with meningitis accompanied by gangrene of the extremities would be our guess) has received a new pair of legs.
A five year old British girl who had her outer limbs amputated due to meningitis (meningococcemia with meningitis accompanied by gangrene of the extremities would be our guess) has received a new pair of legs.







